Method for soluble expression of recombinant protein of human brain natriuretic peptide and application
A technology of human brain natriuretic peptide and recombinant protein, which is applied to the preparation method of peptides, peptide/protein components, animal/human proteins, etc., can solve the problems of bacterial cell rupture, and achieve the effect of easy repeatability and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1 BNP codon optimization
[0086] Synthetic DsbA mut -wtBNP (SEQ ID No11) and DsbA mut -optBNP (SEQ ID No3) gene sequence (synthesized by Invitrogen), and the His-Tag histidine tag sequence and the thrombin cleavage site sequence were introduced into the gene fragment respectively, and introduced at the 5' end of the artificially synthesized gene fragment Nco Ⅰ Endonuclease site, introduced at the 3' end Hind III endonuclease site. The synthetic fragment and the pET28a(+) vector were subjected to Nco Ⅰ and Hind Ⅲ Double enzyme digestion, T4 DNA ligase to connect gene fragments and vector fragments, routinely transform DH5α competent cells, use kanamycin resistance to screen to obtain positive clones, extract plasmids and carry out gene sequencing, and retain the correct clones and plasmids.
[0087] The wtBNP gene sequence is (SEQ ID No 1):
[0088] 5’-AGC CCC AAG ATG GTG CAA GGG TCT GGC TGC TTT GGG AGG AAG ATG GAC CGG ATC AGC TCC TCC AGT GGC CTG GGC...
Embodiment 2
[0094] Embodiment 2 Recombinant Vector and Engineering Bacteria Construction
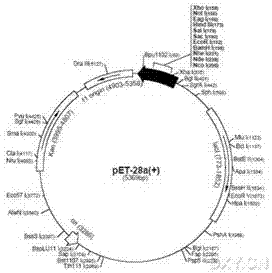
[0095] Commissioned Invitrogen to synthesize His-DsbA mut -Thrombin recognition site-BNP sequence (SEQ ID No 3), and introduced at the 5' end of the gene fragment Nco Ⅰ Endonuclease site, introduced at the 3' end Hind III endonuclease site. The synthetic gene fragment and pET28a (+) vector ( figure 1 ) respectively via Nco Ⅰ and Hind Ⅲ double enzyme digestion, T4 DNA ligase to connect the gene fragment and the vector fragment, routinely transform DH5α competent cells (Tiangen Biochemical Technology (Beijing) Co., Ltd.), screen positive clones according to kanamycin resistance, and extract plasmids. Recombinant plasmid via Nco Ⅰ and Hind Ⅲ Double enzyme digestion and agarose gel electrophoresis identification, and at the same time entrust Invitrogen to sequence the recombinant plasmid, use BioEdit software to analyze the sequencing results, the results are the same as the designed sequ...
Embodiment 3
[0097] Embodiment three engineering bacteria fermentation
[0098] 1. Take pET-28a-DsbA mut -BNP / BL21(DE3) seed solution, inoculated in LB liquid medium (containing 50 μg / mL kanamycin) at 1:500 at 37°C, 150 rpm for overnight culture;
[0099] 2. Measure the OD of the overnight culture solution 600 for 4 o'clock. It was inoculated into a fermenter containing 30 L of TB medium at a ratio of 1:20. The dissolved oxygen was controlled at 30%, the pH was controlled at 7.0, and cultured at 37°C. The stirring speed of the fermenter was automatically associated with the dissolved oxygen. Regular sampling, measure OD 600 value;
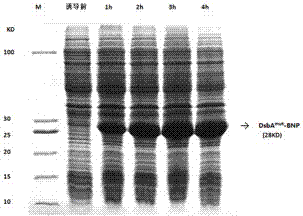
[0100] 3. When the culture solution OD 600 When it reaches 12, add IPTG (final concentration 0.5 mM) to the fermenter, control the dissolved oxygen to 30%, control the pH to 7.0, culture and induce at 37°C, take samples every hour to measure OD 600 value;
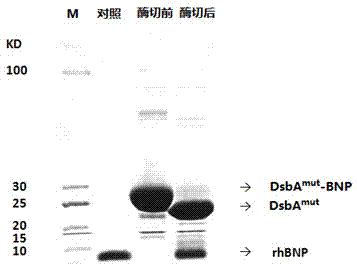
[0101] 4. After the engineered bacteria were induced for 4 hours, the fermentation culture was s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| Elution gradient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com