Compound paracetamol and amantadine hydrochloride capsule and preparation method thereof
A compound paracetamol and capsule technology, applied in the field of medicine, can solve the problems of low bioavailability of the human body, unsatisfactory drug disintegration, poor drug stability, etc., and achieves unaffected therapeutic efficacy, small weight difference, and drug resistance. Ingredient stabilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
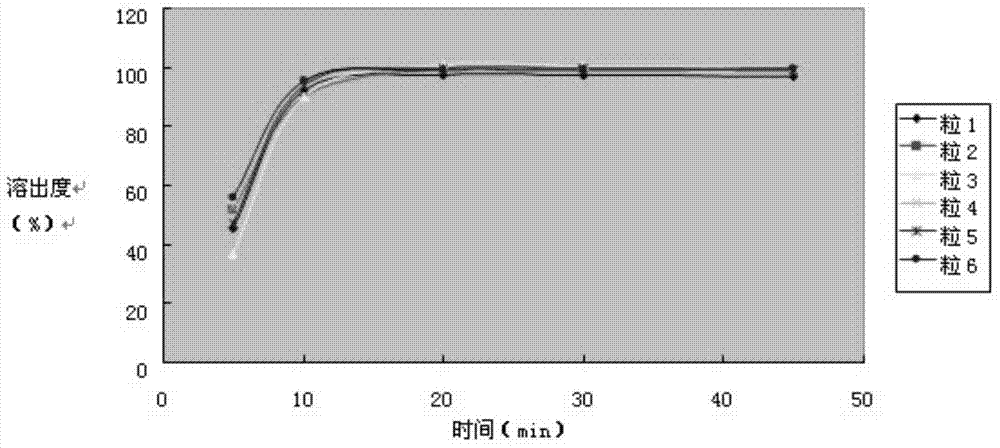
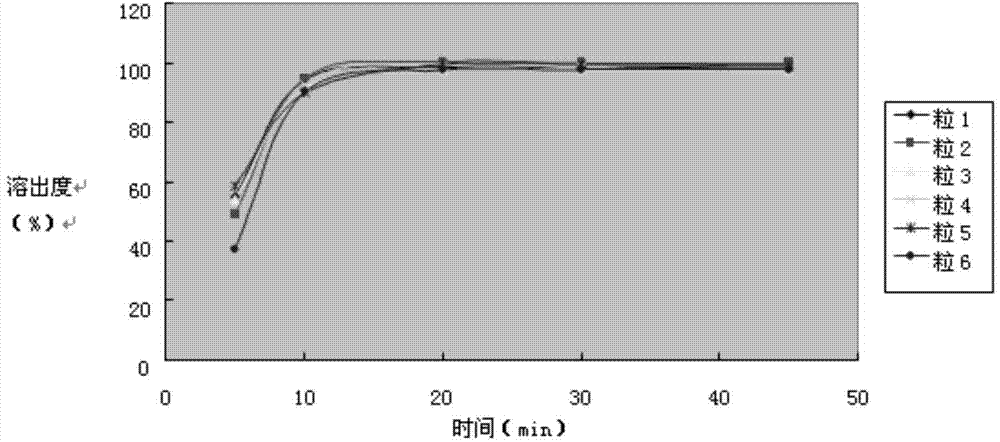
Examples
Embodiment 1
[0089] The preparation of embodiment 1 compound acetaminophen capsules:
[0090] Compound paracetamol capsules are made from the following raw materials in parts by mass:
[0091]
[0092] The preparation method is as follows:
[0093] (1) Paracetamol, amantadine hydrochloride, caffeine, chlorpheniramine maleate, crushed through an 80-mesh sieve for later use, artificial bezoar through an 80-mesh sieve for later use, montmorillonite through a 350-mesh sieve; sucrose through a 100-mesh sieve spare;
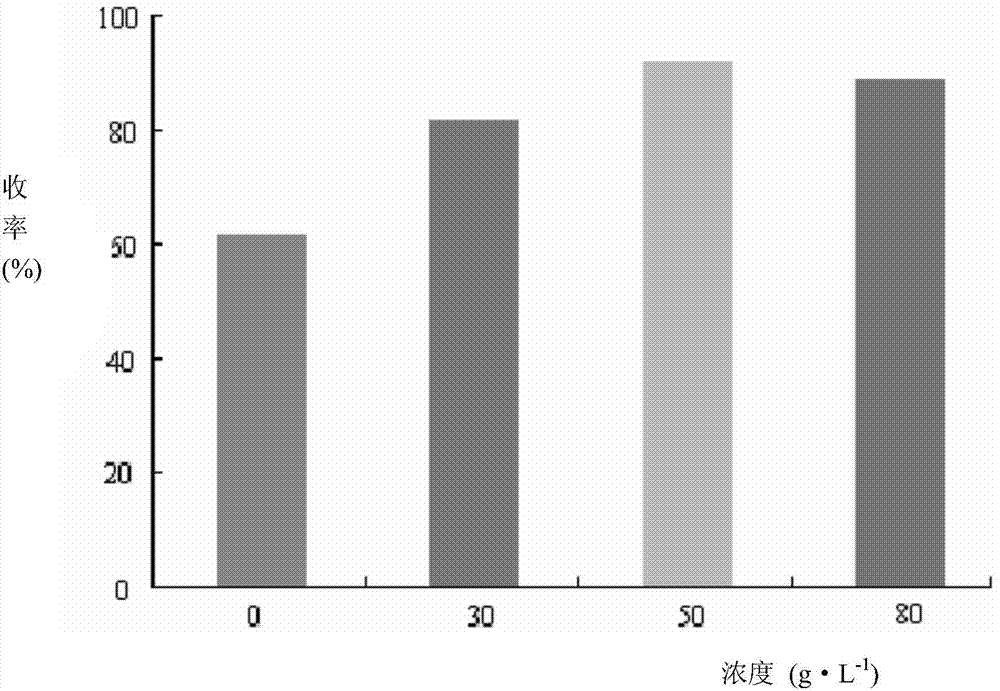
[0094] (2) Take sucrose and montmorillonite according to the proportioning ratio, first dissolve sucrose in boiling water, then add montmorillonite, stir evenly, and prepare 50g L-1 montmorillonite / sucrose solution for subsequent use;
[0095] (3) Take by weighing paracetamol, amantadine hydrochloride, caffeine, chlorpheniramine maleate, artificial bezoar and mix uniformly, then add the montmorillonite / sucrose solution prepared in step (2), stir and mix uniformly to prepare i...
Embodiment 2
[0100] The preparation of embodiment 2 compound acetaminophen capsules:
[0101] Compound paracetamol capsules are made from the following raw materials in parts by mass:
[0102]
[0103]
[0104] The preparation method is as follows:
[0105] (1) Paracetamol, amantadine hydrochloride, caffeine, chlorpheniramine maleate, crushed through a 100-mesh sieve for later use, artificial bezoar through a 100-mesh sieve for later use, montmorillonite through a 400-mesh sieve; sucrose through a 110-mesh sieve spare;
[0106] (2) Take sucrose and montmorillonite according to the proportioning ratio, first dissolve sucrose in boiling water, then add montmorillonite, stir evenly, and prepare 50g L-1 montmorillonite / sucrose solution for subsequent use;
[0107] (3) Take by weighing paracetamol, amantadine hydrochloride, caffeine, chlorpheniramine maleate, artificial bezoar and mix uniformly, then add the montmorillonite / sucrose solution prepared in step (2), stir and mix uniformly t...
Embodiment 3
[0112] The preparation of embodiment 3 compound acetaminophen capsules:
[0113] Compound paracetamol capsules are made from the following raw materials in parts by mass:
[0114]
[0115] The preparation method is as follows:
[0116] (1) Paracetamol, amantadine hydrochloride, caffeine, chlorpheniramine maleate, crushed through a 90-mesh sieve for later use, artificial bezoar through a 90-mesh sieve for later use, montmorillonite through a 375-mesh sieve; sucrose through a 100-mesh sieve spare;
[0117] (2) Take sucrose and montmorillonite according to the proportioning ratio, first dissolve sucrose in boiling water, then add montmorillonite, stir evenly, and prepare 45g L-1 montmorillonite / sucrose solution for subsequent use;
[0118] (3) Take by weighing paracetamol, amantadine hydrochloride, caffeine, chlorpheniramine maleate, artificial bezoar and mix uniformly, then add the montmorillonite / sucrose solution prepared in step (2), stir and mix uniformly to prepare into ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com