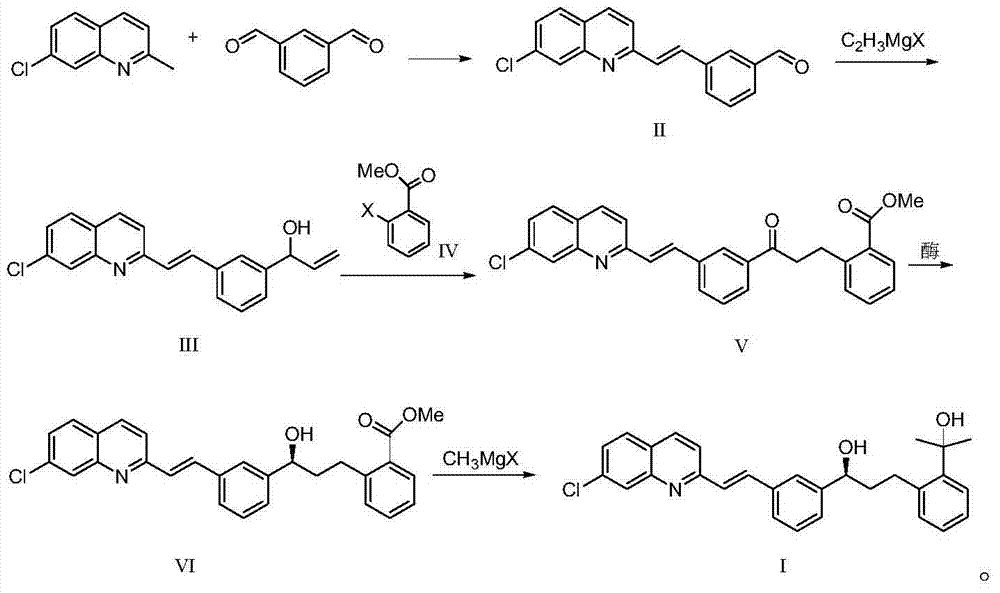
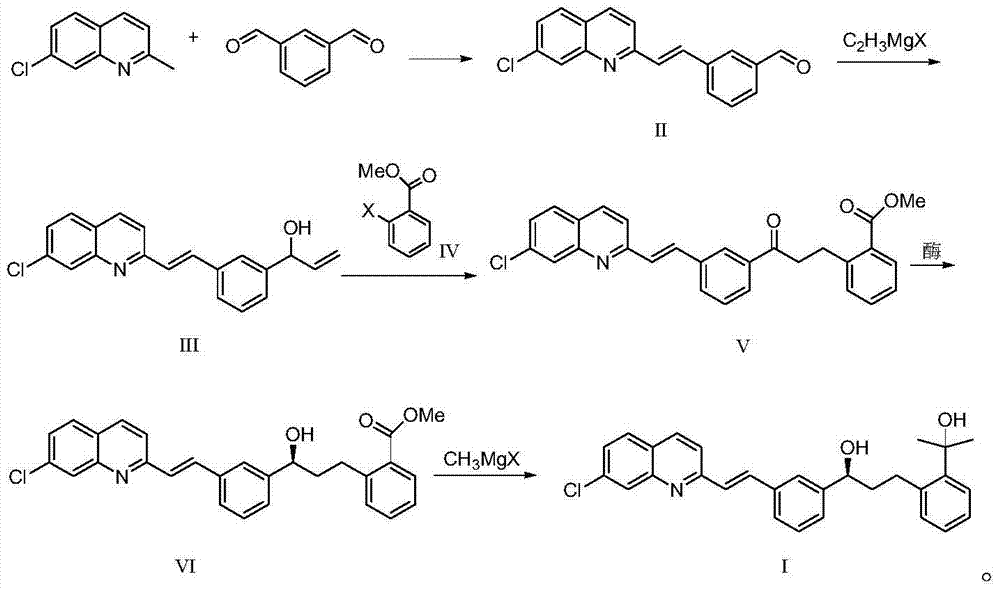
Preparation method for montelukast sodium intermediate
A technology of montelukast sodium and intermediates, which is applied in the field of pharmaceutical engineering and technology, can solve the problems such as recovery and reuse schemes that have not been reported in literature, and achieves stereoselectivity and yield increase, cost reduction, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13-
[0031] The preparation of embodiment 13-[(2E)-2-(7-chloro-2-quinolyl) vinyl] benzaldehyde (formula II)
[0032] Under the protection of nitrogen, put 40g of 7-chloroquinaldine, 80g of isophthalaldehyde, 150g of acetic anhydride, and 102g of triethylamine into the three-necked flask successively, heat up to 100°C, keep the temperature for 8 hours, and detect 7-chloroquinaldine by TLC The response is complete. After the reaction was completed, 270 g of xylene was added and stirred while it was hot, cooled to 10° C. for crystallization for 2 hours, filtered, rinsed with xylene, and the yield was 95%.
Embodiment 21-
[0033] Preparation of Example 21-[3-[(2E)-2-(7-chloro-2-quinolyl)vinyl]phenyl]-2-propen-1-alcohol (Formula III)
[0034] Under the protection of nitrogen, put 12g of 3-[(2E)-2-(7-chloro-2-quinolyl)vinyl]benzaldehyde (Formula II) and 100mL of toluene into the three-necked flask successively, and slowly add 40g of it at 15°C The THF solution of vinylmagnesium chloride was added in about 0.5 hours, and the reaction temperature was controlled at 10°C to 15°C. After the addition, the reaction was kept for 3 hours, and the reaction was detected by TLC. Add the reaction solution to 200g of 10% ammonium acetate aqueous solution, add acetic acid to adjust the pH to 5-6, let stand to separate the phases, concentrate the organic phase, slowly add 35g of 95% ethanol to crystallize, keep warm at 10°C-15°C for 1 hour, and filter with suction The product was obtained with a yield of 91%.
Embodiment 32-3
[0035] Example 32-[3-[3-[(2E)-2-(7-chloro-2-quinolyl)vinyl]phenyl]-3-oxopropyl]benzoic acid methyl ester (Formula V) preparation of
[0036] Under nitrogen protection, drop into 1-[3-[(2E)-2-(7-chloro-2-quinolyl) vinyl] phenyl]-2-propen-1-ol (formula III ) 20g, methyl o-iodobenzoate 18.7g, triethylamine 8.3g, TEBA20g, palladium acetate 0.5g and xylene 200mL. The temperature was raised to 90° C. to 100° C., and the reaction was kept for 9 hours. TLC monitored that the reaction of the raw materials was complete. Cool down to 60°C, filter with suction, concentrate the filtrate under reduced pressure, add about 200mL of 95% ethanol, cool to about 20°C for crystallization for 2 hours, and filter with suction to obtain the product with a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com