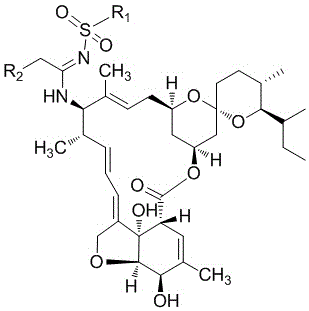
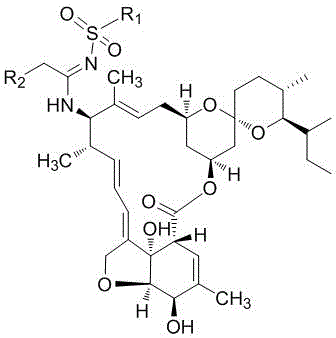
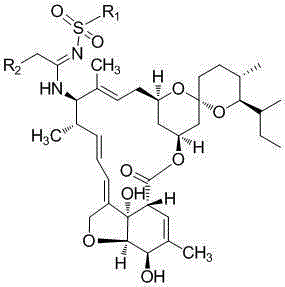
A kind of milbemycin analog and its preparation method and application in pesticide
A technology of milbemycin and analogues, applied in the field of milbemycin analogues and their preparation, can solve the problems of high cost, paralysis and death of insects, limited sources of milbemycin, etc., and achieve reduced synthesis costs and high contact kill active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of (7a)
[0025] The specific process is as follows:
[0026] Synthesis of Intermediate 2: Dissolve 2 g (2.25 mmol) of ivermectin in 39.6 mL of dry methanol, add 0.4 mL of concentrated sulfuric acid dropwise in an ice bath, and react at 0°C for 30 min after the addition is complete , and then stirred at room temperature for about 16 h, TLC detected that the reaction was complete. Diluted with dichloromethane, with 5% NaHCO 3 aqueous solution, and then the aqueous layer was washed with CH 2 Cl 2 After repeated extraction, the organic layers were combined, and the organic layer was washed with water and saturated brine successively. The organic layer was collected, dried with anhydrous magnesium sulfate, filtered and concentrated to obtain a crude product. The crude product was purified by silica gel column chromatography (200-300 mesh) to obtain 1.4 g of the desired pure product.
[0027] Synthesis of Intermediate 3: Dissolve 5.86g (10mmol) of Intermedia...
Embodiment 2
[0034] Synthesis of (7b)
[0035] The experimental procedure is the same as in Example 1, except that p-toluenesulfonyl azide is replaced by p-chlorophenylsulfonyl azide, and phenylacetylene is replaced by p-methylphenylacetylene (CAS: 766-97-2). The detection data of the product obtained by the reaction are as follows:
[0036] Yield: 61%; white solid, melting point: 137-139°C; 1 HNMR (400MHz, CDCl 3 ) δ :7.85(d,2H, J =8.4,Ar-H),7.43(d,2H, J =8.4,Ar-H),7.18(d,2H, J =8.4,Ar-H),7.01(d,2H, J =8.4,Ar-H),5.67-5.70(m,1H,10-H),5.52-5.55(m,1H,9-H),5.30-5.41(m,3H,3-H,11-H, 19-H),4.56-4.67(m,3H,8a-H,15-H),4.38-4.43(m,1H,-CH 2 C=N-),4.25-4.30(m,2H,5-H,-CH 2 C=N-),4.20(s,1H,13-H),3.93(d,1H, J =6.0,6-H),3.89(s,3H,Ar-CH 3 ),3.78(s,1H,7-OH),3.51-3.53(m,1H,17-H),3.20-3.23(m,2H,2-H,25-H),2.62-2.63(m,1H ,12-H),2.17-2.31(m,4H,5-OH,16-H,24-H),1.25-1.87(m,17H,4-Me,14-Me,20-H,26-H ,27-H,22-H,23-H,18-H),0.70-1.19(m,12H,12-Me,27-Me,24-Me,26-Me); MS-ESI m / z :891.3[M+H] + .
Embodiment 3
[0038] Synthesis of (7c)
[0039] The experimental procedure is the same as in Example 1, except that p-toluenesulfonyl azide is replaced by methylsulfonyl azide. The detection data of the product obtained by the reaction are as follows:
[0040] Yield: 49%; white solid, melting point: 133-135 ℃ ; 1 HNMR (400MHz, CDCl 3 ) δ :7.40-7.49(m,2H,Ar-H),7.32-7.39(m,3H,Ar-H),5.67-5.74(m,1H,10-H),5.53-5.55(m,1H,9- H),5.11-5.42(m,3H,3-H,11-H,19-H),4.57-4.67(m,3H,8a-H,15-H),4.40-4.48(m,1H,- CH 2 C=N-),4.27-4.35(m,2H,5-H,-CH 2 C=N-),4.11-4.13(m,1H,13-H),3.92-3.98(m,1H,6-H),3.50(s,3H,-SO 2 CH 3 ),3.20-3.23(m,1H,7-OH),3.07-3.12(m,1H,17-H),3.02-3.04(m,2H,2-H,25-H),2.64-2.65(m ,1H,12-H),2.19-2.32(m,4H,5-OH,16-H,24-H),1.26-1.90(m,17H,4-Me,14-Me,20-H,26 -H,27-H,22-H,23-H,18-H),0.70-1.05(m,12H,12-Me,27-Me,24-Me,26-Me); MS-ESI m / z :781.6[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com