Adamantyl pyridinecarboxamide complex as well as intermediate, preparation method and application thereof
A technology of adamantyl pyridine carboxamide and complexes, which is applied in the direction of copper organic compounds, drug combinations, anti-toxins, etc., and can solve the problems of easy hydrolysis by protease, high cost, and difficult extraction of SOD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
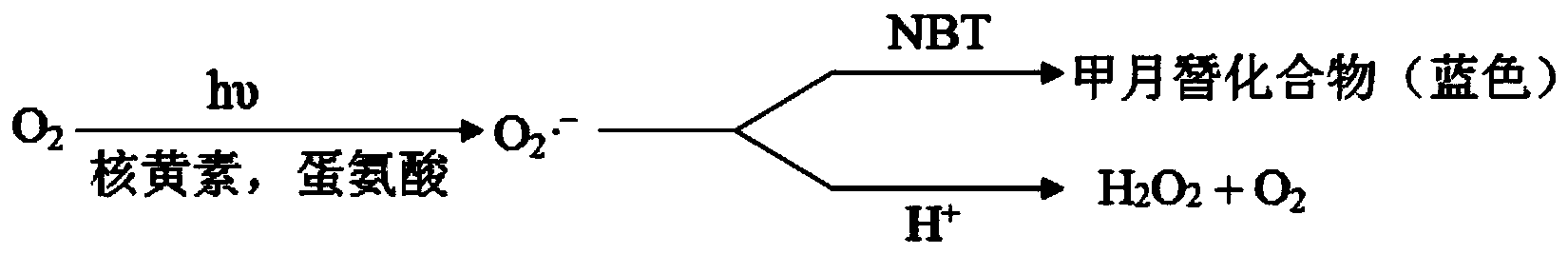
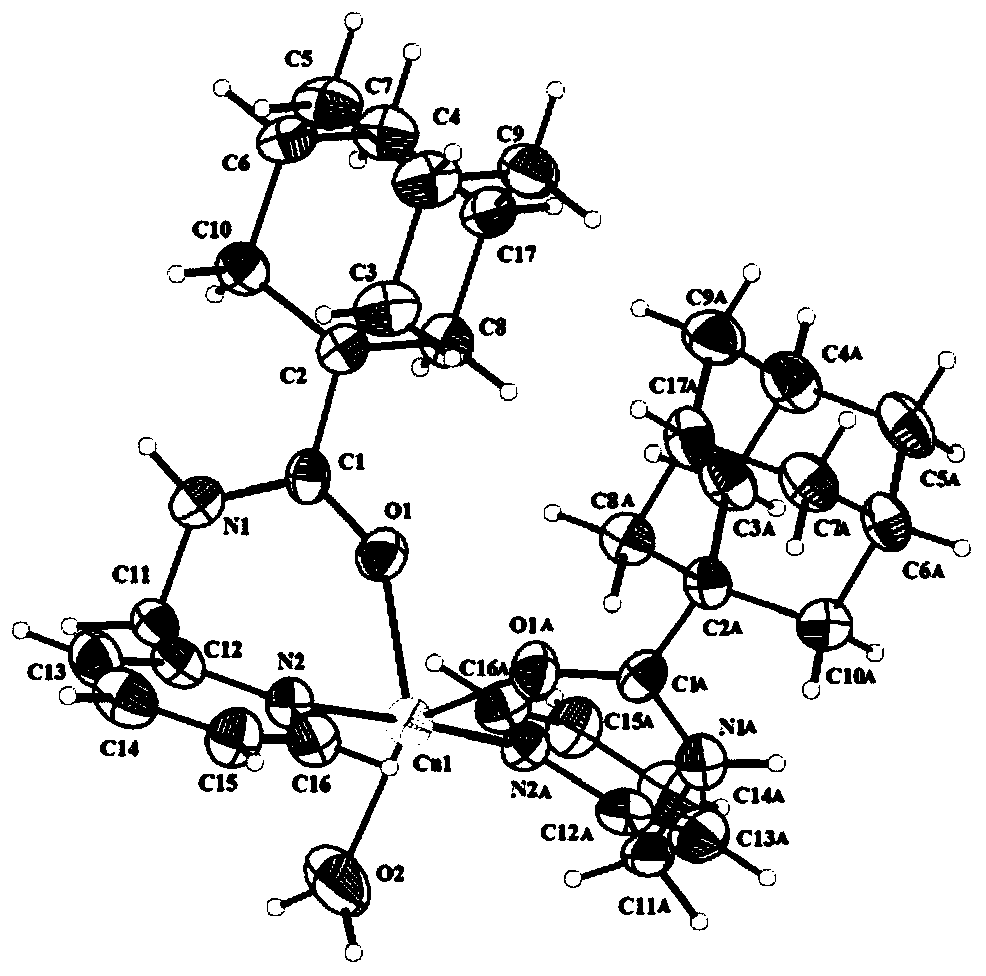
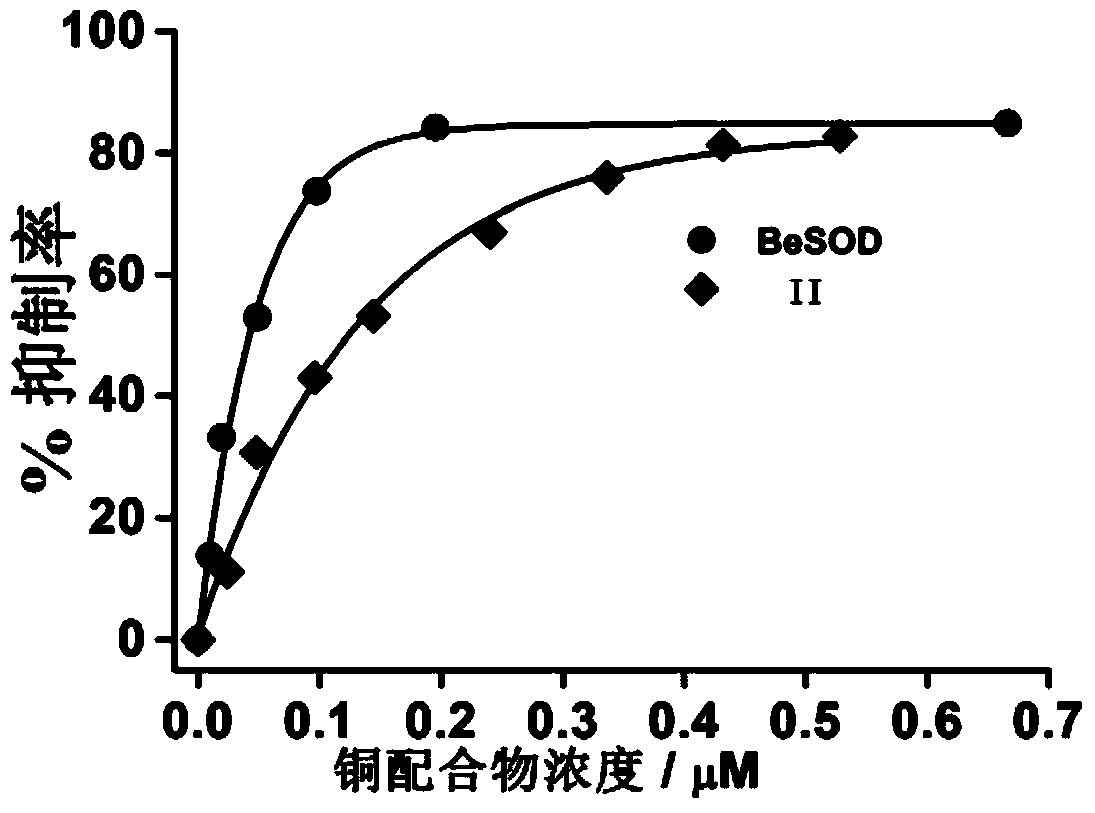
Image
Examples
preparation example Construction
[0054] In the preparation method of the above intermediate, in order to increase the yield of the contact reaction, preferably the contact reaction is carried out below 0°C, and the reaction temperature of the contact reaction is -10-0°C, preferably -3-0°C;
[0055] According to the present invention, in order to make the contact reaction fully proceed, the reaction time of the contact reaction is 3-20 h, preferably 10-15 h.
[0056] [ M(N3) 2 ]·6H 2 O carries out complex reaction;
[0057]
[0058] In formula (L1), n is 1-3, preferably, n is 1;
[0059] In the above formula, M is a divalent metal cation, and N3 is selected from perchlorate ion, hexafluorophosphate ion or tetrafluoroborate ion;
[0060] Preferably, M is Cu 2+ or Zn 2+ , N3 is perchlorate ion;
[0061] More preferably, the [M(N3) 2 ]·6H 2 O is Cu(ClO 4 ) 2 ·6H 2 O.
[0062] According to the present invention, the organic solvent only needs to be an organic solvent capable of dissolving the rea...
preparation example 1
[0072] Preparation of 1-adamantanyl chloride:
[0073] In a 250mL round-bottomed flask, add 10.0mmol of 1-adamantanecarboxylic acid solid and 60mL of anhydrous toluene solvent in sequence, and slowly add 20mL of thionyl chloride dropwise after magnetic stirring to dissolve, reflux at 80°C for 8 hours, and cool to 20°C , the solvent was removed by rotary evaporation, and 10 mL of anhydrous toluene was added three times, and the solvent and excess thionyl chloride were removed by rotary evaporation to obtain a light yellow crystalline powder.
Embodiment 1-1
[0075] The preparation of the intermediate of structure shown in formula (L2):
[0076]
[0077] In a 100mL round-bottomed flask, at 0°C, add 5.0mmol of 1-adamantanyl chloride and 25mL of anhydrous toluene in sequence. After magnetically stirring to dissolve, add 10mmol of anhydrous potassium carbonate, and slowly add 2-methylaminopyridine 6.3 mmol, react under ice-bath conditions for 12 hours, filter, and obtain 1.154 g of light yellow crude product after removing the solvent by rotary evaporation under reduced pressure. The crude product was recrystallized from a mixed solvent of ethyl acetate and ether to obtain a white powder. Recrystallized in a mixed solvent of ethyl acetate and petroleum ether (v / v=1:3) to obtain 1.050 g of white powder with a yield of 76%. Its detection data are as follows.
[0078] The white powder was subjected to elemental analysis, and the result was C 17 h 22 N 2 O: C, 75.36%; N, 10.18%; H, 8.47%; Theoretical calculation value: C, 75.52%; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com