Oral dosage form of lentinan and preparation method thereof
A technology of lentinan and dosage form, which is applied in the field of pharmaceutical dosage form research, can solve the problems of low oral utilization efficiency and large dosage, and achieve the effects of improving bioavailability, wide application, and reducing daily dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
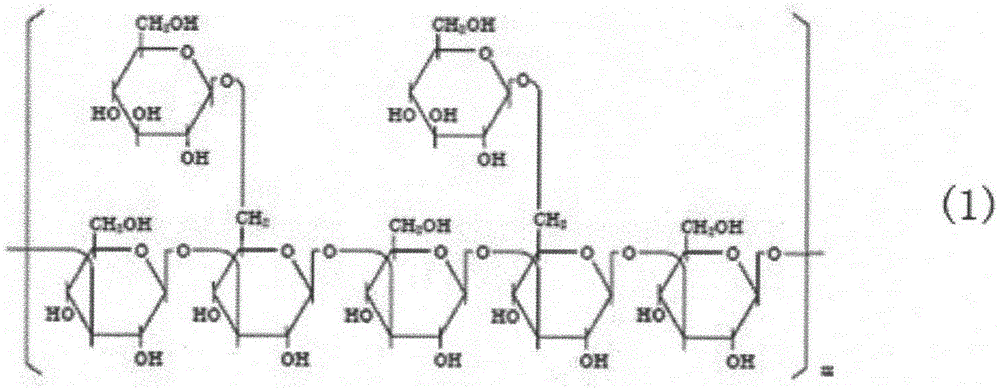
[0025] An oral dosage form of lentinan, which contains pure β and triple-helical lentinan with a weight average molecular weight of 400,000 to 800,000 as shown in formula (1), the purity of which is 96.1 wt%, and the lentinan At 3500~3300cm -1 、2920-2800cm -1 、1100-1000cm -1 、890cm -1 With infrared absorption. Congo red reaction of lentinan was positive (+). The preparation method of the oral dosage form of the above-mentioned lentinan, the specific steps are: in a high-speed mixing granulator, add 99.7g of mannitol, dissolve 0.1g of the above-mentioned lentinan in 50mL water, add it into the mannitol under stirring, and Stir at 15 Hz for 12 minutes, pass through a 20-mesh sieve, and make granules. Dry the granules in a drying oven, add 0.2 g of magnesium stearate, mix evenly, transfer to a tablet machine for tableting, adjust the tablet weight to 0.1 g, and make 1000 tablets to obtain lentinan oral tablets. The obtained lentinan oral tablet has a smooth surface and good...
Embodiment 2
[0027] An oral dosage form of lentinan, which contains pure β and triple helix configuration lentinan with a weight average molecular weight of 400,000 to 800,000 as shown in formula (1), the purity of which is 96.1wt%, and the lentinan is in 3500~3300cm -1 、2920-2800cm -1 、1100-1000cm -1 、890cm -1 With infrared absorption. Congo red reaction of lentinan was positive (+). The preparation method of the oral dosage form of above-mentioned lentinan, concrete steps are: in high-speed mixing granulator, add microcrystalline cellulose 40g and mannitol 57.9g, above-mentioned lentinan 0.1g and glucose 2g are dissolved in 50mL water, in Add it into microcrystalline cellulose and mannitol under stirring, stir at 15 Hz for 15 minutes, pass through a 20-mesh sieve, and make granules. The granules are dried in a drying oven, sized through a 20-mesh sieve, mixed evenly, and then transferred into a capsule filling machine, and each capsule is filled with 0.1g to obtain 1000 lentinan ora...
Embodiment 3
[0029] An oral dosage form of lentinan, which contains pure β and triple-helical lentinan with a weight average molecular weight of 400,000 to 800,000 as shown in formula (1), the purity of which is 96.1 wt%, and the lentinan At 3500~3300cm -1 、2920-2800cm -1 、1100-1000cm -1 、890cm -1 With infrared absorption. Congo red reaction of lentinan was positive (+). The preparation method of the oral dosage form of the above-mentioned lentinan, the specific steps are: in the boiling granulator, add microcrystalline cellulose 2700g, open the compressed air, make the powder fluidization; Dissolve the above-mentioned lentinan 1g and glucose 299g in 500mL water , pumped into the fluidized granulator under stirring, added by top spray, boiled and granulated for 30 minutes, the air outlet was controlled at 70°C, dried for 5 minutes, the material was passed through a 40-mesh sieve to make granules, and transferred to the granule packaging machine for sub-packaging , 3g per pack, made in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com