Small-molecule modified target paclitaxel precursor medicament, as well as preparation method and application thereof
A prodrug, paclitaxel technology, applied in the field of biomedicine, can solve the problems such as insurmountable immunotoxicity, achieve the effect of avoiding the toxicity of the body's immunogen, improving the therapeutic effect, and requiring less production environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This embodiment is a preparation method of a targeted paclitaxel prodrug modified by a small molecule, comprising the following steps:
[0028] (1) Dissolve paclitaxel (100mg, 0.117mmol) in 15ml of dichloromethane (dissolve completely with a rotor), then add arginine (75.82mg, 0.117mmol) and stir (with turbidity), add a small amount of DMAP (0.117mmol), When it becomes turbid and clear, change the ice bath and stir, and add 10ml of dichloromethane dissolved in EDC·HCl (44.85mg, 0.234mmol) dropwise with a syringe. After adding dropwise for 20 minutes, stir at room temperature for 22 hours. TLC spot plate tracking once, after the reaction, the amino acid-paclitaxel reaction solution was obtained;
[0029] (2) Add the same volume of dichloromethane (15ml) to the reaction solution of step (1) to dilute, and wash twice with the same volume of water (40ml), then wash twice with the same volume of saturated saline (40ml), and use a separate The dichloromethane solution in whi...
Embodiment 2
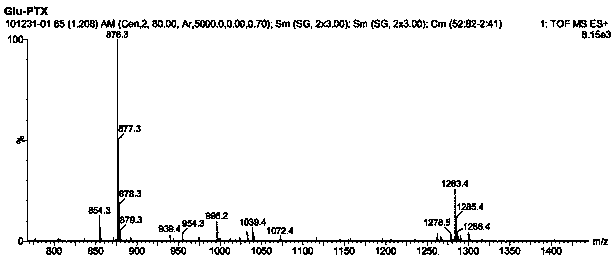
[0039] In step (1) of this example, paclitaxel (100mg, 0.117mmol) was dissolved in 15ml of dichloromethane, and glutamic acid (49.78mg, 0.117mmol) was added to stir the reaction. In step (6), aminofluorescein Instead of FITC, other steps are the same as in Example 1 to obtain folic acid-glutamic acid-paclitaxel prodrugs and folic acid-glutamic acid-paclitaxel-aminofluorescein prodrugs, wherein the LC of the intermediate product glutamic acid-paclitaxel conjugate - MS chromatogram as Figure 4 As shown, the yield was 92%.
[0040] Folic acid-amino acid-paclitaxel-fluorescent dye prodrug water solubility determination of embodiment 1 and 2:
[0041] Accurately weigh 100 mg of the paclitaxel prodrug of Examples 1 and 2, add 50 μL of water each time with a micro-syringe, observe the dissolution situation by direct observation, until it is too much, count the amount of water required to dissolve 100 mg of the paclitaxel prodrug, and calculate the solubility. Wherein, the solubili...
Embodiment 3
[0043] In this example, the amino acid is methionine, and the fluorescent dye is a near-infrared fluorescent dye. Other steps are the same as in Example 1 to obtain folic acid-methionine-paclitaxel prodrugs and folic acid-methionine-paclitaxel-near-infrared fluorescent dye prodrugs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com