New thienopyridine compound
A technology for compounds and diseases, used in organic chemistry, drug combinations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
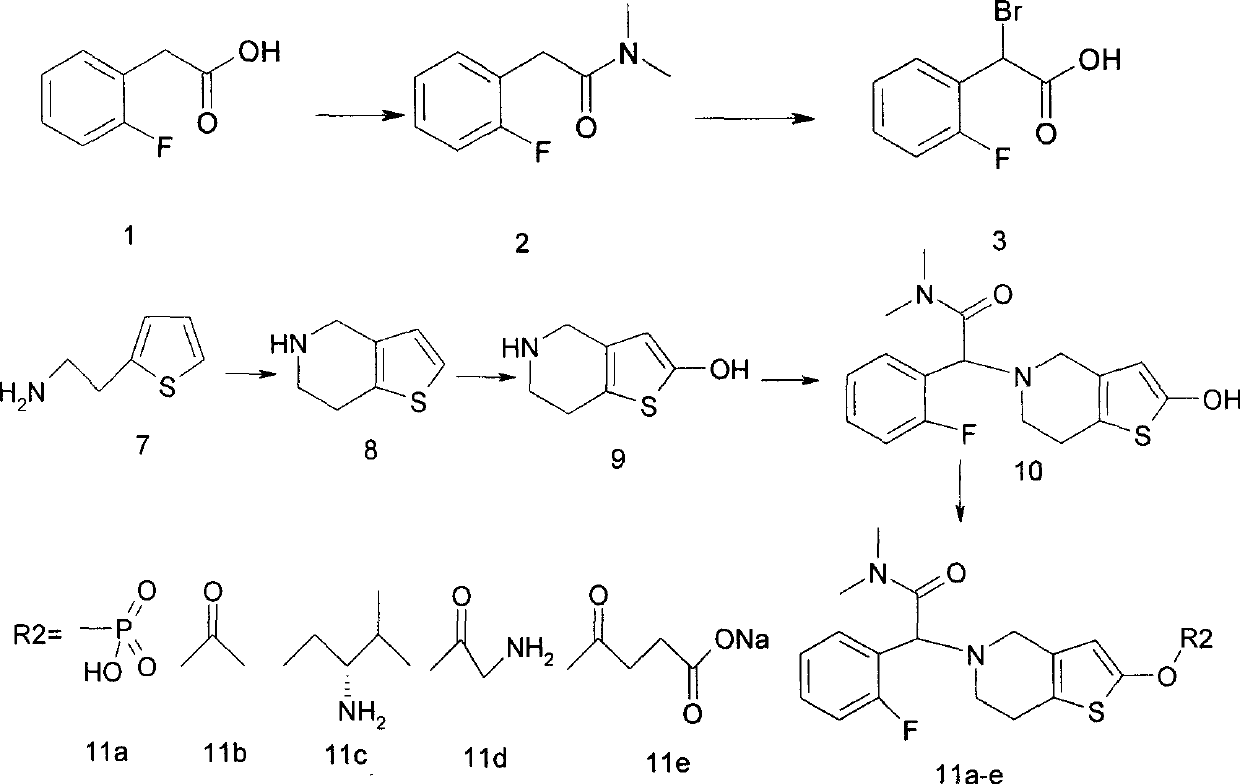
[0019] Embodiment 1: the preparation of each compound:
[0020] Because the compounds described herein have strong coherence, in order to describe the preparation method of each compound in detail, accurately and conveniently, it is expressed with 1 embodiment, and the serial number of the compound is indicated below each compound of the following synthetic route, for more Concise descriptions are replaced by serial numbers in the following preparation methods:
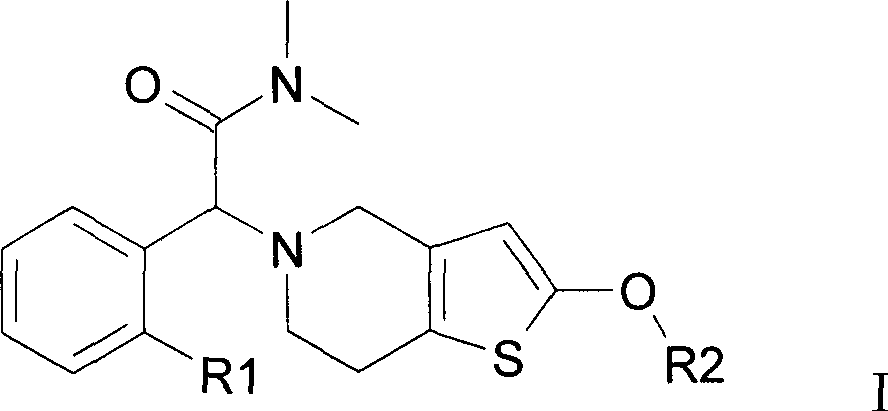
[0021]
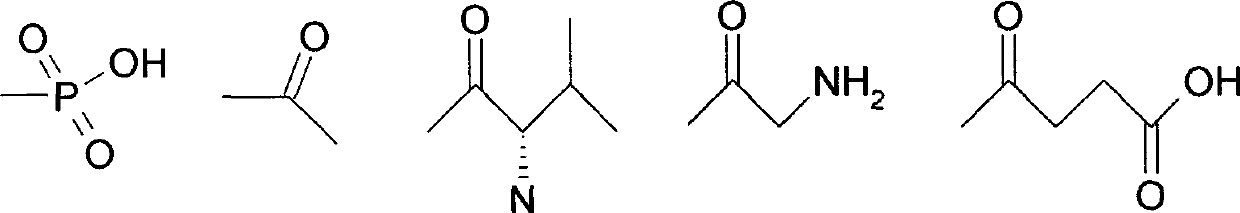
[0022] Different compounds 11a, 11b, 11c, 11b and 11e are obtained when R is a different group in the above scheme.
[0023] (1), the synthesis of compound 2:
[0024] Dissolve 154 grams of compound 1 and 220 grams of triethylamine in 500 mL of THF, add 142 grams of thionyl chloride dropwise under ice-cooling, complete the dropwise addition within 30 minutes, continue the reaction for 30 minutes, and then move the reaction solution to room temperature React for one hour, then reflux for another hour, coo...
Embodiment 2
[0043] In the same way, R 1 -Cl, R 2 Each compound when it is a different organic acid or inorganic acid. Example 2: Effects on Cerebral Infarction Volume in Rats with Focal Cerebral Ischemia
[0044] (1) Experimental materials and methods
[0045] Wistar rats, body weight 250-280g. They were reared separately before and after the operation, the room temperature was kept at 23-25°C, and they had their own food and water. The tMCAO model was prepared according to the method of Longa et al. The rats were anesthetized with 10% chloral hydrate (350mg / kg, i.p.), the body temperature was maintained at 37±0.5°C, and they were fixed on the operating table in a supine position. The skin was cut along the midline of the neck, and the right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were carefully separated. Cut the ECA ligation and straighten it to be in line with the ICA. Cut a small opening on the ECA, and insert a 4.0cm-long, ...
Embodiment 13
[0056] Example 13: Effects of compound 10, compound 11a and 11b on sleep in rats:
[0057] (1) Sleep improvement test
[0058] Animal source: Kunming white mice, 18-22 grams, male, clean animals provided by Guangdong Experimental Animal Center. The temperature of the experimental animal breeding room is 22±2°C, the relative humidity is 50-70%, and the animal feed is provided by Guangdong Experimental Animal Center.
[0059] In this experiment, three groups of compounds 10, 11a, and 11b were set up at 25 mg / kg respectively, and a distilled water control group was also set up.
[0060] Sample treatment: Take 25mg of each sample and add distilled water to 20ml to make a uniform suspension for testing.
[0061] Way of giving samples: gavage
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com