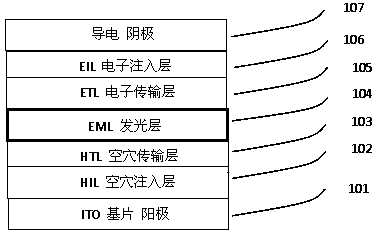
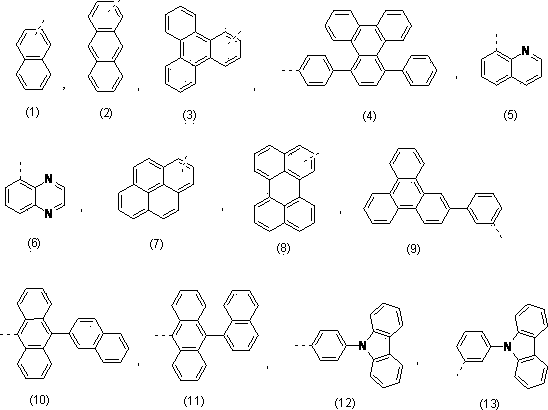
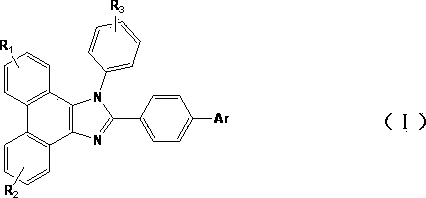
Blue organic light emitting diode material
A light-emitting diode, an organic technology, applied in the direction of light-emitting materials, organic chemistry, electrical components, etc., can solve the problems of low external quantum efficiency, etc., and achieve the goals of improving charge injection and light-emitting stability, long-life luminous performance, and high-efficiency luminous performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of Compound 22
[0052] .
[0053] (1) Synthesis of intermediate M1
[0054]
[0055] In a 500 mL three-necked flask with a thermometer and a condenser tube, add 10 g (48 mmol) of phenanthrenequinone, 8.9 g (48 mmol) of p-bromobenzaldehyde, 5.38 g (57.7 mmol) of aniline, and 37.06 g (480 mmol) of ammonium acetate and 200ml glacial acetic acid, N 2 Replaced three times, raised the temperature to 130°C and refluxed the reaction overnight. The reaction was monitored by TLC plate until complete. Cool to room temperature, filter, pour the filter residue into 200ml of methanol, stir and wash twice, filter to obtain the desired product, and receive 19.8g (92%) of a tan solid. MS=448, mp=255°C, HPLC: 99.5%.
[0056] (2) Synthesis of intermediate M2
[0057] In a 250 mL three-necked flask, sequentially add intermediate M1 (9 g, 20 mmol), bis(pinacolate) diboron (7.62 g, 30 mmol), CH 3 COOK (5.9g, 60mmol), 1,4-dioxane 125 mL and S-phos (1.2g, 3mmol),...
Embodiment 2
[0069] Synthesis of compound 25
[0070] .
[0071] (1) Synthesis of intermediate M7
[0072] In a 250mL three-necked flask, add carbazole 16.72g, m-bromoiodobenzene 42.44g, cuprous iodide 1.9g, 1,2-diaminocyclohexane 3.42g, anhydrous potassium phosphate 42.4g and solvent 1 , 4-dioxane 160mL, N 2 Replaced three times, raised the temperature to reflux, and stopped after 16 hours of reaction. Cool to room temperature, filter, collect the filtrate, separate by column chromatography (dichloromethane:n-hexane=1:1-1:0), and receive 22g (69%) of the target solid product. M / Z=322.
[0073] (2) Synthesis of intermediate M8
[0074] In a 500 mL three-necked flask, intermediate M7 (20 g, 62.1 mmol), bis(pinacolate) diboron (23.6 g, 93.2 mmol), CH 3 COOK (17.5g, 178.2mmol), 1,4-dioxane 300 mL and S-phos (5.35g, 13.04mmol), N2 replacement three times, adding Pd 2 (dba) 3 (3.98g, 4.35mmol) and heated to reflux, the reaction was stopped after 24h. Cool to room temperature, f...
Embodiment 3
[0078] Synthesis of compound 26
[0079] .
[0080] (1) Synthesis of intermediate M9
[0081] In a 250mL three-necked flask, add carbazole (8.36g, 50mmol), p-bromoiodobenzene (21.22g, 75mmol), cuprous iodide (0.95g, 5mmol), 1,2-diaminocyclohexane (1.71g, 15mmol), anhydrous potassium phosphate (21.2g, 100mmol) and solvent 1,4-dioxane 160mL, N 2 Replaced three times, raised the temperature to reflux, and stopped after 16 hours of reaction. Cool to room temperature, filter, collect the filtrate, separate by column chromatography (dichloromethane:n-hexane=1:1-1:0), and receive 13.4g (83.2%) of the target solid product. M / Z=322.
[0082] (2) Synthesis of intermediate M10
[0083] In a 500 mL three-necked flask, intermediate M9 (20 g, 62.1 mmol), bis(pinacolate) diboron (23.6 g, 93.2 mmol), CH 3 COOK (17.5g, 178.2mmol), 1,4-dioxane 300 mL and S-phos (5.35g, 13.04mmol), N2 replacement three times, adding Pd 2 (dba) 3 (3.98g, 4.35mmol) and heated to reflux, the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com