Pyrazole amide and pyrazole imine derivatives containing substituted 1, 3, 4-thiadiazole thioether as well as preparation method and application of derivatives
A technology of thiadiazole sulfide and pyrazole amide is applied in the field of pyrazole amide and pyrazole imine derivatives and their preparation, which can solve the problems of indistinct bactericidal activity in vitro and achieve a reasonable synthesis route , the effect of simple operation and low cost of synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
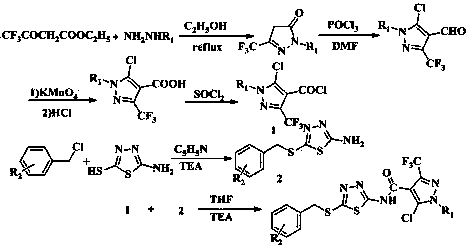
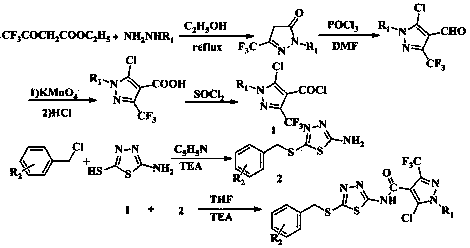
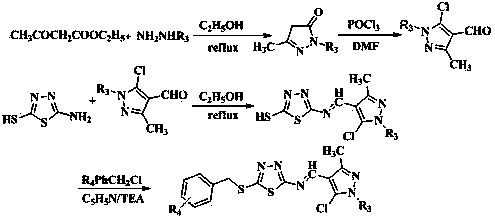
[0091] Example 1: 5-chloro-N-(5-((3-fluorobenzyl)thio)-1,3,4-thiadiazol-2-yl)-1-methyl-3-(trifluoro methyl)-1 H -pyrazole-4-carboxamide (I 1 ) preparation;
[0092] (1) Preparation of 1-methyl-3-trifluoromethyl-5-pyrazolone intermediate:
[0093] Throw 0.1 mol of methylhydrazine into a 250 mL three-necked flask, add 10 mL of absolute ethanol, stir and heat to 50 0 At about C, add 0.1 mol ethyl trifluoroacetoacetate drop by drop, heat to reflux for 5 h, cool to crystallize, filter under reduced pressure, and dry to obtain a yellow solid; yield: 85.8%.
[0094] (2) Preparation of 1-methyl-3-trifluoromethyl-5-chloro-4-pyrazole aldehyde intermediate:
[0095] Add 0.35 mol DMF to a 250 mL three-neck flask, and slowly add 0.83 mol POCl dropwise at 0 °C 3 , after dropping, stirred for 20 min, slowly added 0.10 mol 1-methyl-3-trifluoromethyl-5-pyrazolone, raised the temperature to 80-90 °C for 5 h, cooled and slowly poured into 200 mL of ice water, Stand for 2 h, filter with suc...
Embodiment 2
[0104] Example 2: 5-chloro-N-(5-((3-fluorobenzyl)thio)-1,3,4-thiadiazol-2-yl)-1-phenyl-3-(trifluoro methyl)-1 H -pyrazole-4-carboxamide (I 2 ) preparation;
[0105] (1) Preparation of 1-phenyl-3-trifluoromethyl-5-pyrazolone intermediate:
[0106] Throw 0.1 mol of phenylhydrazine into a 250 mL three-neck flask, add 10 mL of absolute ethanol, stir and heat to 50 0 At around C, add 0.1 mol ethyl trifluoroacetoacetate dropwise, heat to reflux for 5 h, cool to crystallize, filter under reduced pressure, and dry to obtain a yellow solid; yield: 83.8%.
[0107] (2) Preparation of 1-phenyl-3-trifluoromethyl-5-chloro-4-pyrazole aldehyde intermediate:
[0108] Add 0.35 mol DMF to a 250 mL three-neck flask, and slowly add 0.83 mol POCl dropwise at 0 °C 3 After dropping, stir for 20 min, slowly add 0.10 mol 1-phenyl-3-trifluoromethyl-5-pyrazolone, heat up to 80-90 °C for 5 h, cool down and slowly pour into 200 mL of ice water, Stand for 2 h, filter with suction, wash with water, an...
Embodiment 3
[0117] Example three: 5-chloro-N-(5-((4-nitrobenzyl)thio)-1,3,4-thiadiazol-2-yl)-1-methyl-3-(tri Fluoromethyl)-1 H -pyrazole-4-carboxamide (I 3 ) preparation;
[0118] (1) Preparation of 1-methyl-3-trifluoromethyl-5-pyrazolone intermediate:
[0119] It was synthesized according to the method and conditions of Example 1 (1).
[0120] (2) Preparation of 1-methyl-3-trifluoromethyl-5-chloro-4-pyrazole aldehyde intermediate:
[0121] It was synthesized according to the method and conditions of Example 1 (2).
[0122] (3) Preparation of 1-methyl-3-trifluoromethyl-5-chloro-4-pyrazole acid intermediate:
[0123] It was synthesized according to the method and conditions of Example 1 (3).
[0124] (4) Preparation of 1-methyl-3-trifluoromethyl-5-chloro-4-pyrazole chloride intermediate:
[0125]It was synthesized according to the method and conditions of Example 1 (4).
[0126] (5) Preparation of 2-amino-5-(4-nitro)benzyl-1,3,4-thiadiazole sulfide intermediate:
[0127] Add 20mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com