Preparation method for clopidogrel hydrogen sulphate
A technology of clopidogrel bisulfate and clopidogrel free base, which is applied in the field of preparation of clopidogrel bisulfate to achieve the effects of convenient operation, simple post-processing, and avoiding double-tethering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
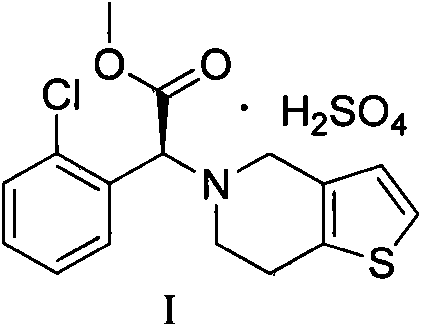
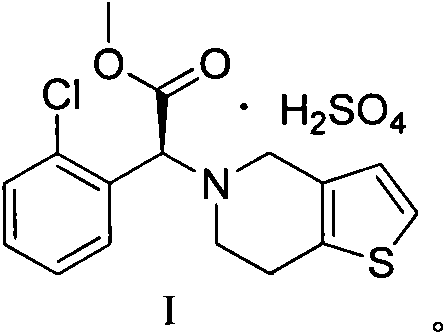
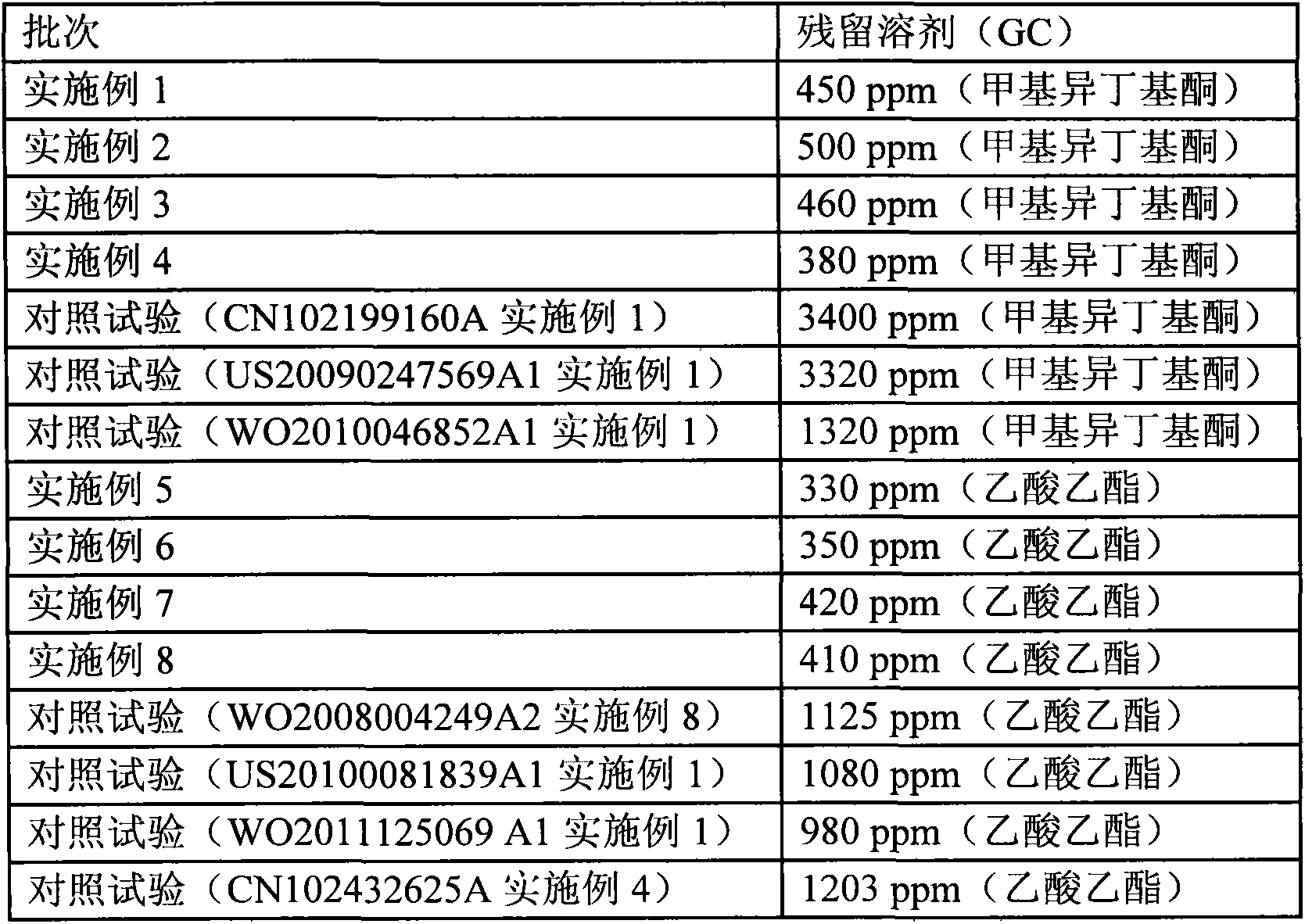
[0026] Example 1: Dissolve 10 g of concentrated sulfuric acid in 990 g of methyl isobutyl ketone solution, cool the solution to 20° C. after completely dissolving, then slowly add clopidogrel free base methyl isobutyl ketone with a mass concentration of 50% The solution was 63g, and the addition time was 0.5h. Stir while adding, and a white solid was precipitated. After the addition, the reaction solution was heated to 15°C, and stirred at this temperature for 3h, filtered, and dried to obtain clopidogrel hydrogen sulfate, which was obtained by XRD It was detected as clopidogrel bisulfate crystal form I, the yield was 88%, the purity was 99.8%, mp: 184~186°C, [α] D =55.0°, residual solvent (GC): 450 ppm.
Embodiment 2
[0027] Example 2: Dissolve 10 g of concentrated sulfuric acid in 323 g of methyl isobutyl ketone solution, cool the solution to 20° C. after completely dissolving, then slowly add clopidogrel free base methyl isobutyl ketone with a mass concentration of 40% The solution was 75g, and the dropwise addition time was 1h, stirring while adding, and a white solid was precipitated. After the addition, the reaction solution was heated to 20°C, and continued to stir at this temperature for 5h, filtered, and dried to obtain clopidogrel hydrogen sulfate, which was obtained by XRD It was detected as clopidogrel bisulfate crystal form I, the yield was 90%, the purity was 99.6%, mp: 184~186℃, [α] D =55.0°, residual solvent (GC): 500 ppm.
Embodiment 3
[0028] Example 3: Dissolve 10 g of concentrated sulfuric acid in 24 g of methyl isobutyl ketone solution, cool the solution to 20° C. after completely dissolving, then slowly add clopidogrel free base methyl isobutyl ketone with a mass concentration of 30% The solution was 73g, and the dropwise addition time was 2h. Stirring while adding, precipitated a white solid. After the addition, the reaction solution was heated to 30°C, and continued to stir at this temperature for 10h, filtered, and dried to obtain clopidogrel hydrogen sulfate, which was obtained by XRD It was detected as clopidogrel bisulfate crystal form I, the yield was 85%, the purity was 99.8%, mp: 184~186℃, [α] D =55.0°, residual solvent (GC): 460 ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com