Monosialotetrahexosylganglioside sodium preparation and preparation method thereof
A technology of sodium ganglioside and monosialic acid, which is used in nervous system diseases, pharmaceutical formulations, medical preparations containing active ingredients, etc. problems, to achieve the effects of convenient clinical use, avoidance of secondary pollution, and simple storage conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
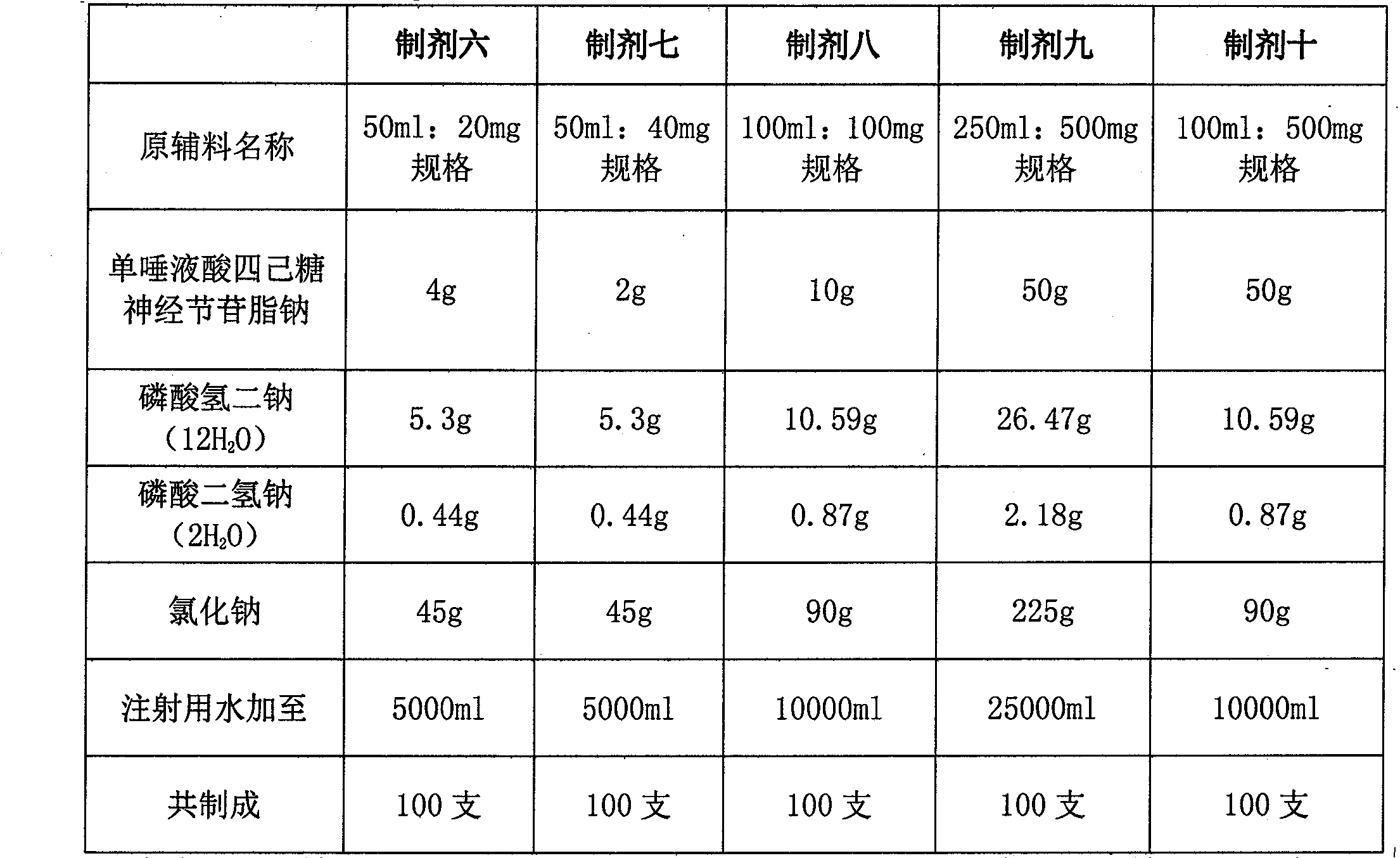
[0020] Example 1 Preparation 1 (pH7.42):
[0021] Name of raw material
[0022] Co-made
[0023] Preparation process: Weigh monosialotetrahexosyl ganglioside sodium, disodium hydrogen phosphate (anhydrous), sodium dihydrogen phosphate (anhydrous) and sodium chloride in the prescribed amount, add about 95% of the total volume Dissolve in water for injection, add water for injection to full volume. Filter through a 0.22 μm microporous membrane, fill in a 50ml low-borosilicate soda-lime glass bottle, melt seal, and sterilize by autoclaving at 121°C for 15 minutes.
Embodiment 2
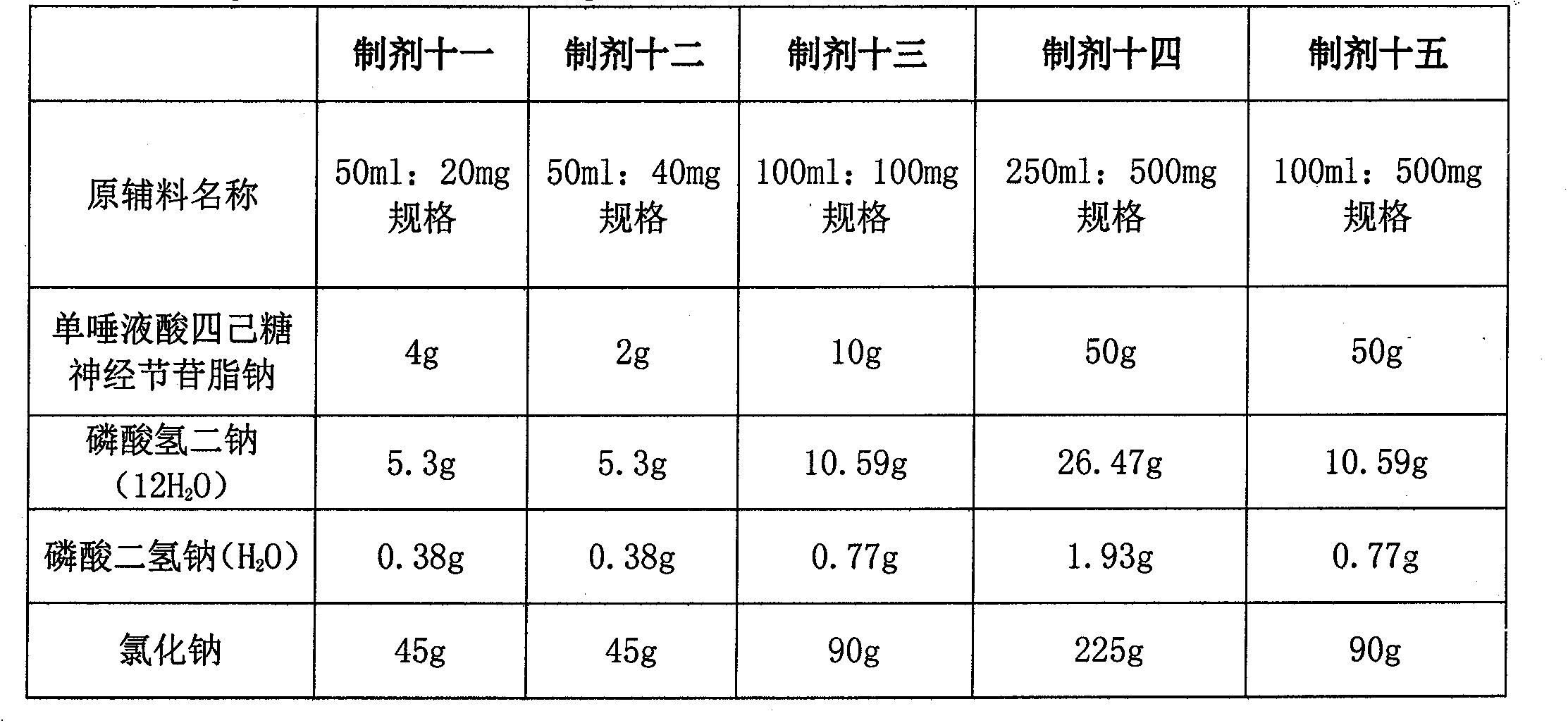
[0024] Example 2 Preparation 2 (pH7.48):
[0025] Name of raw material
[0026] Preparation process: Weigh monosialotetrahexosyl ganglioside sodium, disodium hydrogen phosphate (anhydrous), sodium dihydrogen phosphate (anhydrous) and sodium chloride in the prescribed amount, add about 95% of the total volume Dissolve in water for injection, add water for injection to full volume. Filter through a 0.22 μm microporous membrane, fill in a 50ml low-borosilicate soda-lime glass bottle, melt seal, and sterilize by autoclaving at 121°C for 15 minutes.
Embodiment 3
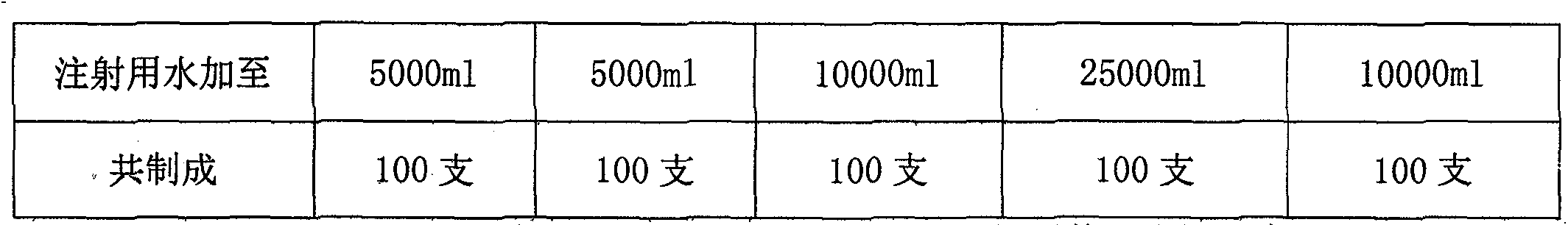
[0027] Example 3 Preparation three (pH7.55):
[0028] Name of raw material
[0029] Preparation process: Weigh monosialotetrahexosyl ganglioside sodium, disodium hydrogen phosphate (anhydrous), sodium dihydrogen phosphate (anhydrous) and sodium chloride in the prescribed amount, add about 95% of the total volume Dissolve in water for injection, add water for injection to full volume. Filter through a 0.22 μm microporous membrane, fill in a 100ml low borosilicate soda calcium glass bottle, melt seal, and sterilize by autoclaving at 121°C for 15 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com