(2-hydroxy-3-dehydroabieticoxy) propyl chitosan-oligosaccharide and preparation method thereof
A technology of dehydroabietyloxy and chitooligosaccharides, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve problems such as limited application, poor solubility, and impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
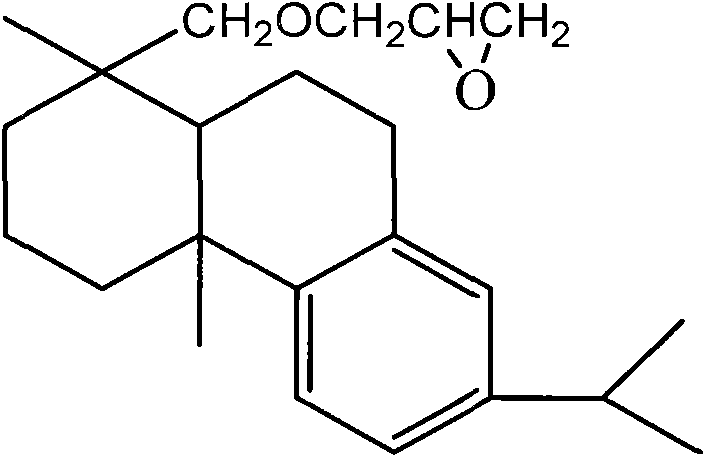
[0021] (2-hydroxyl-3-dehydroabietyloxy) propyl chitooligosaccharides involved in the invention and preparation method thereof, is characterized in that a kind of figure 1 The (2-hydroxyl-3-dehydroabietyloxy) propyl group of the shown structure is a new type of green nonionic surfactant (2-hydroxyl-3-dehydroabietyloxy) propyl group as lipophilic group and chitooligosaccharide group as hydrophilic group (2-hydroxyl-3-dehydroabietyloxy) (hydroabietyloxy) propyl chitooligosaccharides, including preparation of dehydroabietyl glycidyl ether, preparation of water-soluble chitooligosaccharides and (2-hydroxy-3-dehydroabietyloxy) propyl chitooligosaccharides Glycans were prepared using a process consisting of three main processes.
[0022] (1) Preparation of dehydroabietyl glycidyl ether: in BF 3 -In the presence of ether, after dehydroabietyl alcohol directly reacts with epichlorohydrin to obtain 3-chloro-2-hydroxypropyl dehydroabietyl ether, then in the presence of sodium hydroxide ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com