O-imidazolate-n-trimethyl chitosan quaternary ammonium salt and its preparation method and application
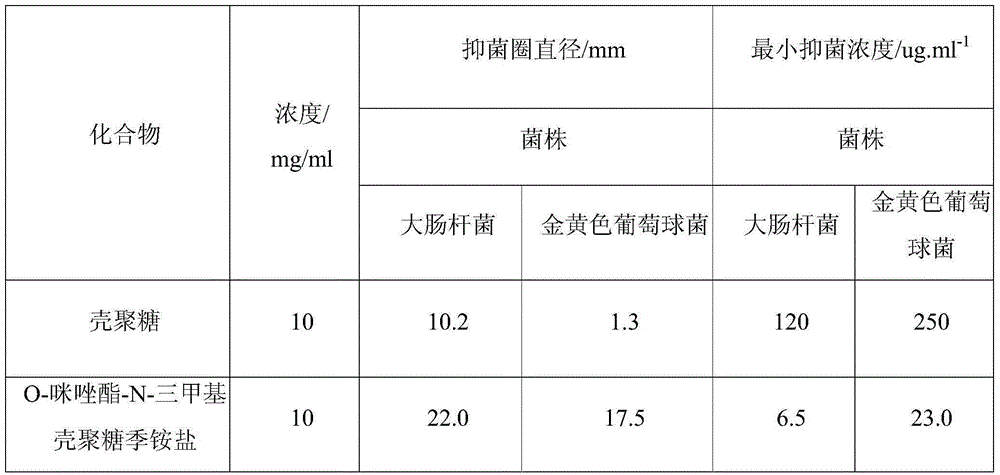
A technology of trimethyl chitosan and imidazolate, which is applied in the field of polymer compound materials, can solve the problems of insolubility in water, achieve the effect of enhancing antibacterial activity and hydrophilicity, good antibacterial performance, and expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
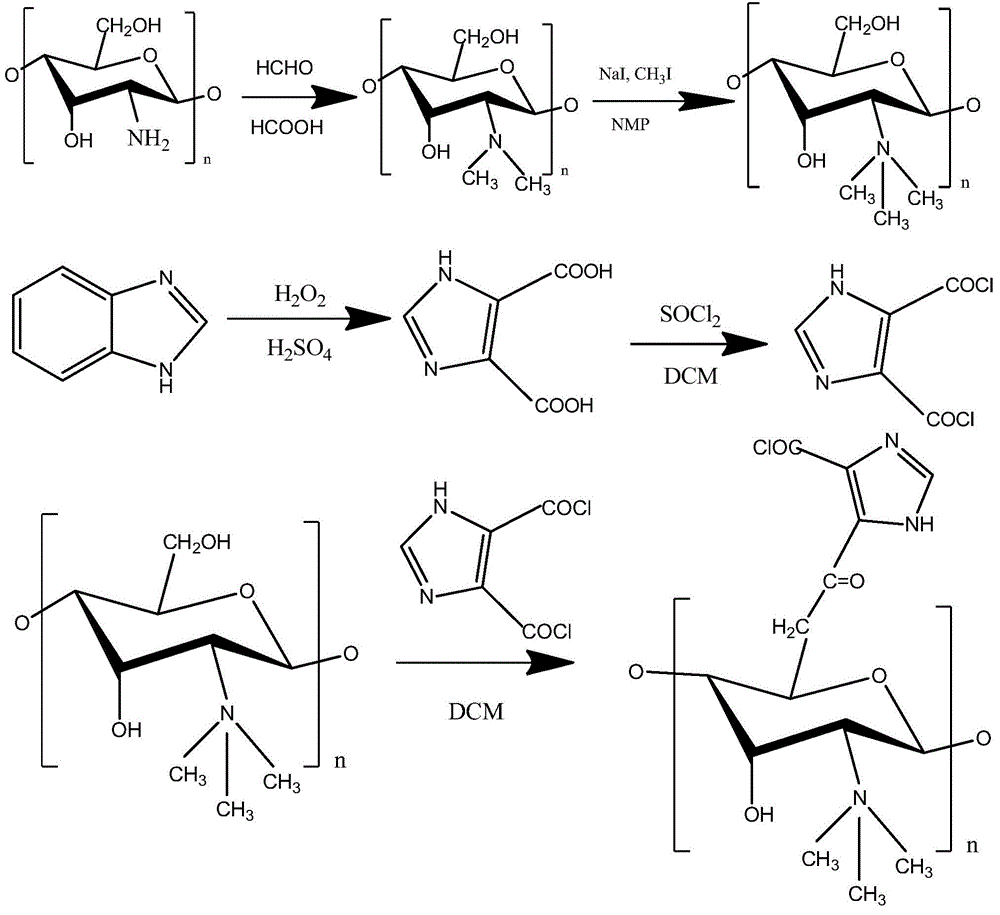
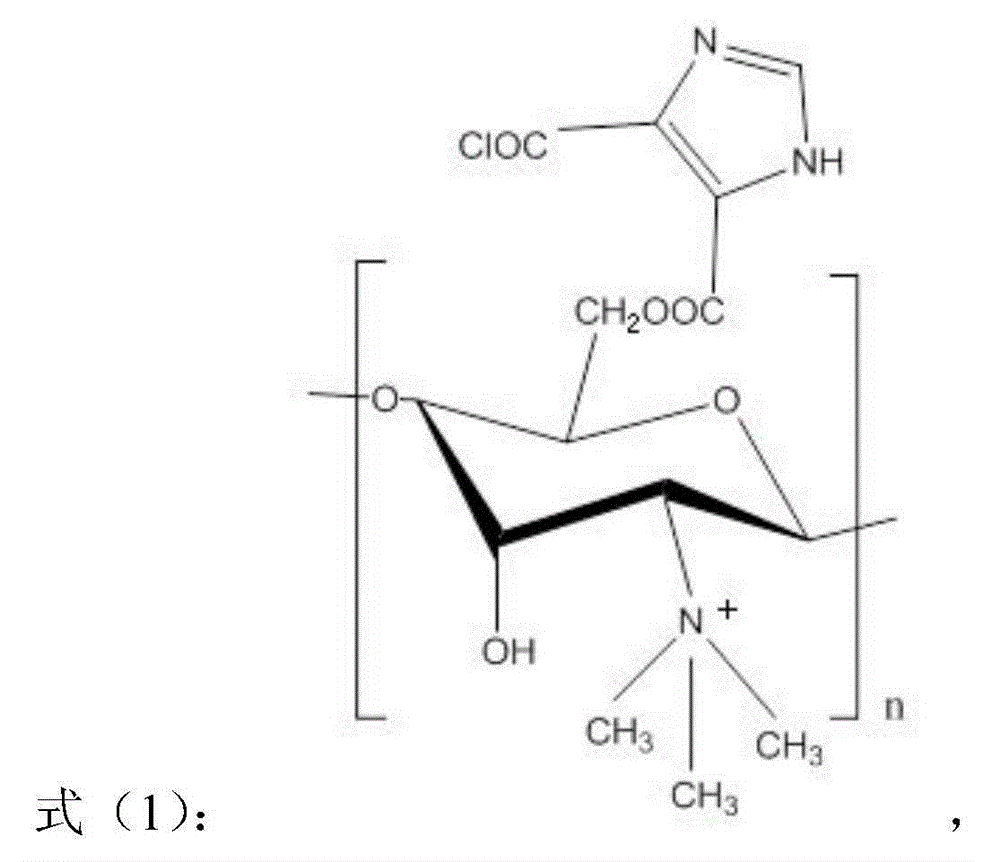
[0029] A kind of O-imidazolate-N-trimethyl chitosan quaternary ammonium salt, its preparation method is as follows:
[0030] (1) Dissolve 4g of chitosan in 200mL of deionized water and 15mL of formic acid, stir and heat up to 70°C, add 10mL of formaldehyde solution, mix well and transfer to a microwave normal pressure reactor, adjust the microwave power to 400W React at 30°C for 240min; adjust pH to alkaline with 15% NaOH solution, filter with suction, wash with water until neutral, add to 50mL N-methyl-2-pyrrolidone after drying, add 40mL methyl iodide, 40°C React for 120 hours; add twice the volume of V (ether) / V (ethanol) = 1:1 mixed solvent for precipitation, use deionized water to dialyze and then freeze-dry to obtain N,N,N-trimethyl chitosan.
[0031] The quaternization degree of N,N,N-trimethyl chitosan was 76% through X-ray photoelectron spectroscopy (XPS) detection of the sample.
[0032] (2) Pipette 5g of benzimidazole into 100mL of concentrated sulfuric acid under ...
Embodiment 2
[0035] A kind of O-imidazolate-N-trimethyl chitosan quaternary ammonium salt, its preparation method is as follows:
[0036](1) Dissolve 4g of chitosan in 200mL of deionized water and 15mL of formic acid, stir and heat up to 70°C, add 10mL of formaldehyde solution, mix well and transfer to a microwave normal pressure reactor, adjust the microwave power to 500W React at 40°C for 180min; adjust the pH to alkaline with 15% NaOH solution, filter with suction, wash with water until neutral, add to 50mL N-methyl-2-pyrrolidone after drying, add 40mL methyl iodide, 40°C React for 120 hours; add twice the volume of V (ether) / V (ethanol) = 1:1 mixed solvent for precipitation, use deionized water to dialyze and freeze-dry to obtain N,N,N-trimethyl chitosan.
[0037] The quaternization degree of N,N,N-trimethyl chitosan was 75% through X-ray photoelectron spectroscopy (XPS) detection of the sample.
[0038] (2) Pipette 5g of benzimidazole into 100mL of concentrated sulfuric acid under ic...
Embodiment 3
[0041] A kind of O-imidazolate-N-trimethyl chitosan quaternary ammonium salt, its preparation method is as follows:
[0042] (1) Dissolve 4g of chitosan in 200mL of deionized water and 15mL of formic acid, stir and heat up to 70°C, add 10mL of formaldehyde solution, mix well and transfer to a microwave normal pressure reactor, adjust the microwave power to 600W React at 50°C for 120 minutes; adjust the pH to alkaline with 15% NaOH solution, filter with suction, wash with water until neutral, add to 50mL N-methyl-2-pyrrolidone after drying, add 40mL methyl iodide, 40°C React for 120 hours; add twice the volume of V (ether) / V (ethanol) = 1:1 mixed solvent for precipitation, use deionized water to dialyze and freeze-dry to obtain N,N,N-trimethyl chitosan.
[0043] The quaternization degree of N,N,N-trimethyl chitosan was 75.2% through X-ray photoelectron spectroscopy (XPS) detection of the sample.
[0044] (2) Pipette 5g of benzimidazole into 100mL of concentrated sulfuric acid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| degree of esterification | aaaaa | aaaaa |
| degree of esterification | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com