Red light organic electrophosphorescence material metal iridium coordination compound and preparation method thereof, and organic electroluminescent device
A phosphorescent material, metal iridium technology, applied in luminescent materials, electro-solid devices, organic chemistry and other directions, can solve the problem of rarely achieving the color purity of dark red and dark green light, reduce the probability of self-quenching, prepare The effect of easy process control and broad commercial development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
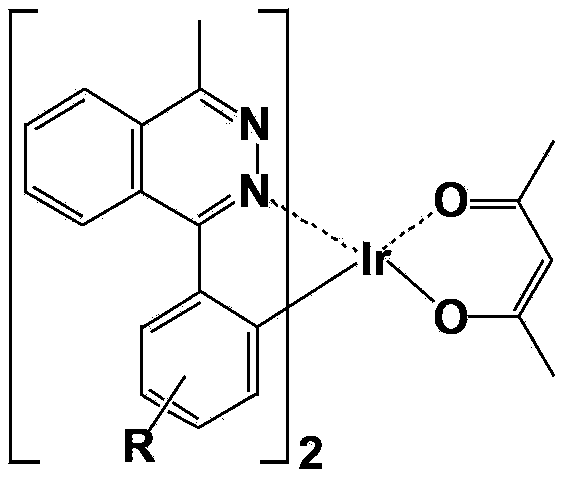
[0059] A red-light organic electrophosphorescent material metal iridium complex bis[1-methyl-4-phenylphthalazine-N,C 2 '] (acetylacetonate) iridium, as shown in the following structural formula:
[0060]
[0061] The preparation method of the metal iridium complex of the above-mentioned red light organic electrophosphorescent material comprises the following steps:
[0062] (1) Synthesis of cyclometallic main ligand 1-methyl-4-phenylphthalazine, the reaction formula is:
[0063]
[0064] The specific operation is:
[0065] Compound A represented by the following structural formula, i.e. 1-methyl-4-chlorophthalazine, and compound B1, i.e. phenylboronic acid are provided respectively:
[0066]
[0067] Under the protection of nitrogen, 0.72g (4.0mmol) of compound A, 0.59g (4.8mmol) of compound B1 and 0.23g (0.20mmol) of tetrakis (triphenylphosphine) palladium were mixed and dissolved in 15mL of DMF, and the concentration of 1mol was added dropwise to 15mL / L of potas...
Embodiment 2
[0098] A red-light organic electrophosphorescent material metal iridium complex bis[1-methyl-4-(6'-methylphenyl)phthalazine-N,C 2 '] (acetylacetonate) iridium, as shown in the following structural formula:
[0099]
[0100] The preparation method of the metal iridium complex of the above-mentioned red light organic electrophosphorescent material comprises the following steps:
[0101] (1) Synthesis of cyclometallic main ligand 1-methyl-4-(2'-methylphenyl)phthalazine, the reaction formula is:
[0102]
[0103] The specific operation is:
[0104] Compound A represented by the following structural formula, i.e. 1-methyl-4-chlorophthalazine, and compound B2, i.e. 2-methylphenylboronic acid are provided respectively:
[0105]
[0106] Under nitrogen protection, 0.72g (4.0mmol) of compound A, 0.68g (5mmol) of compound B2 and 0.11g (0.16mmol) of dichlorobis(triphenylphosphine)palladium were mixed and dissolved in 15mL of toluene, and the concentration of 10mL was added dro...
Embodiment 3
[0127] A red-light organic electrophosphorescent material metal iridium complex bis[1-methyl-4-(5'-methylphenyl)phthalazine-N,C 2 '] (acetylacetonate) iridium, as shown in the following structural formula:
[0128]
[0129] The preparation method of the metal iridium complex of the above-mentioned red light organic electrophosphorescent material comprises the following steps:
[0130] (1) Synthesis of cyclometallic main ligand 1-methyl-4-(3'-methylphenyl)phthalazine, the reaction formula is:
[0131]
[0132] The specific operation is:
[0133] Compound A represented by the following structural formula, i.e. 1-methyl-4-chlorophthalazine, and compound B3, i.e. 3-methylphenylboronic acid are provided respectively:
[0134]
[0135] Under the protection of nitrogen, mix 0.72g (4.0mmol) compound A, 0.65g (4.8mmol) compound B3 and 0.08g (0.12mmol) dichlorobis(triphenylphosphine)palladium in 20mL DMF, and add dropwise 8mL concentration 1 mol / L potassium carbonate aqueous...
PUM
| Property | Measurement | Unit |
|---|---|---|
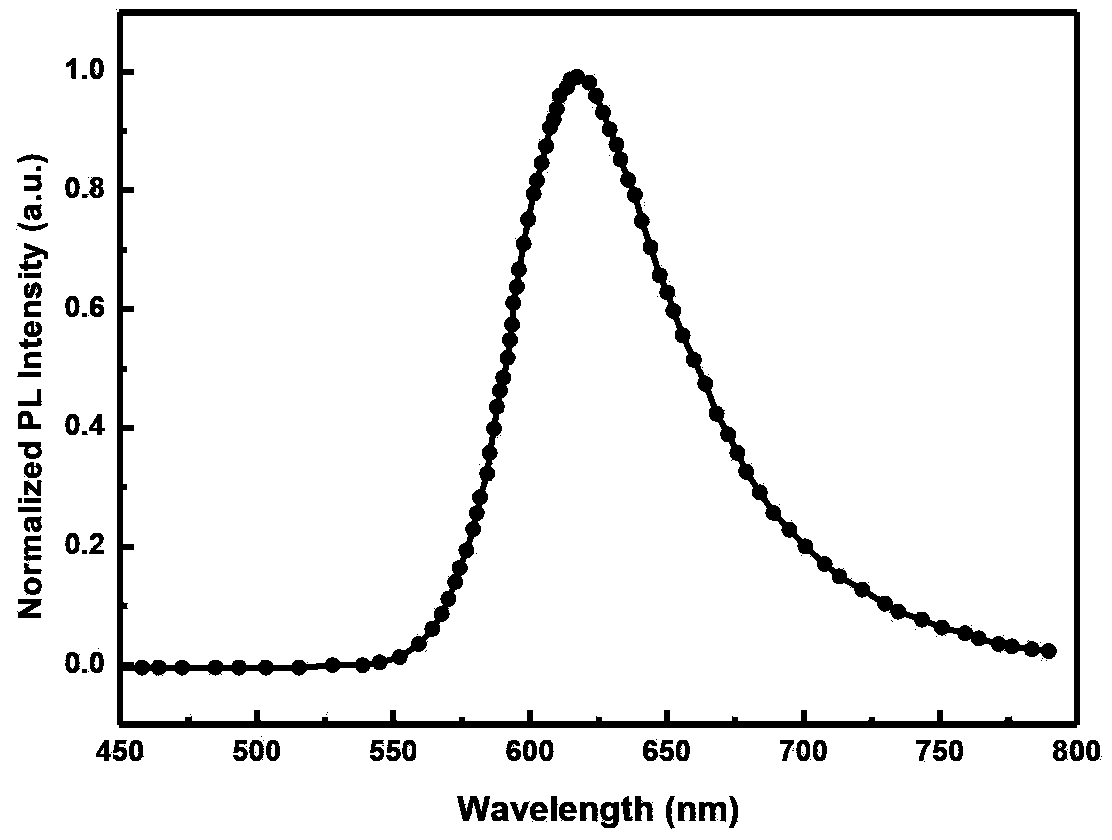
| Maximum luminescence wavelength | aaaaa | aaaaa |
| Maximum luminescence wavelength | aaaaa | aaaaa |
| Maximum luminescence wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com