Synthetic method of ionic liquid catalyzed propylene carbonate to synthesize dimethyl carbonate
A technology of dimethyl carbonate and propylene carbonate is applied in the synthesis field of ionic liquid catalyzing propylene carbonate to synthesize dimethyl carbonate, which can solve the problems of reduced propylene glycol yield, entrained propylene glycol, and large amount of catalyst, so as to reduce the The effect of using cost, production process simplification, and production cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
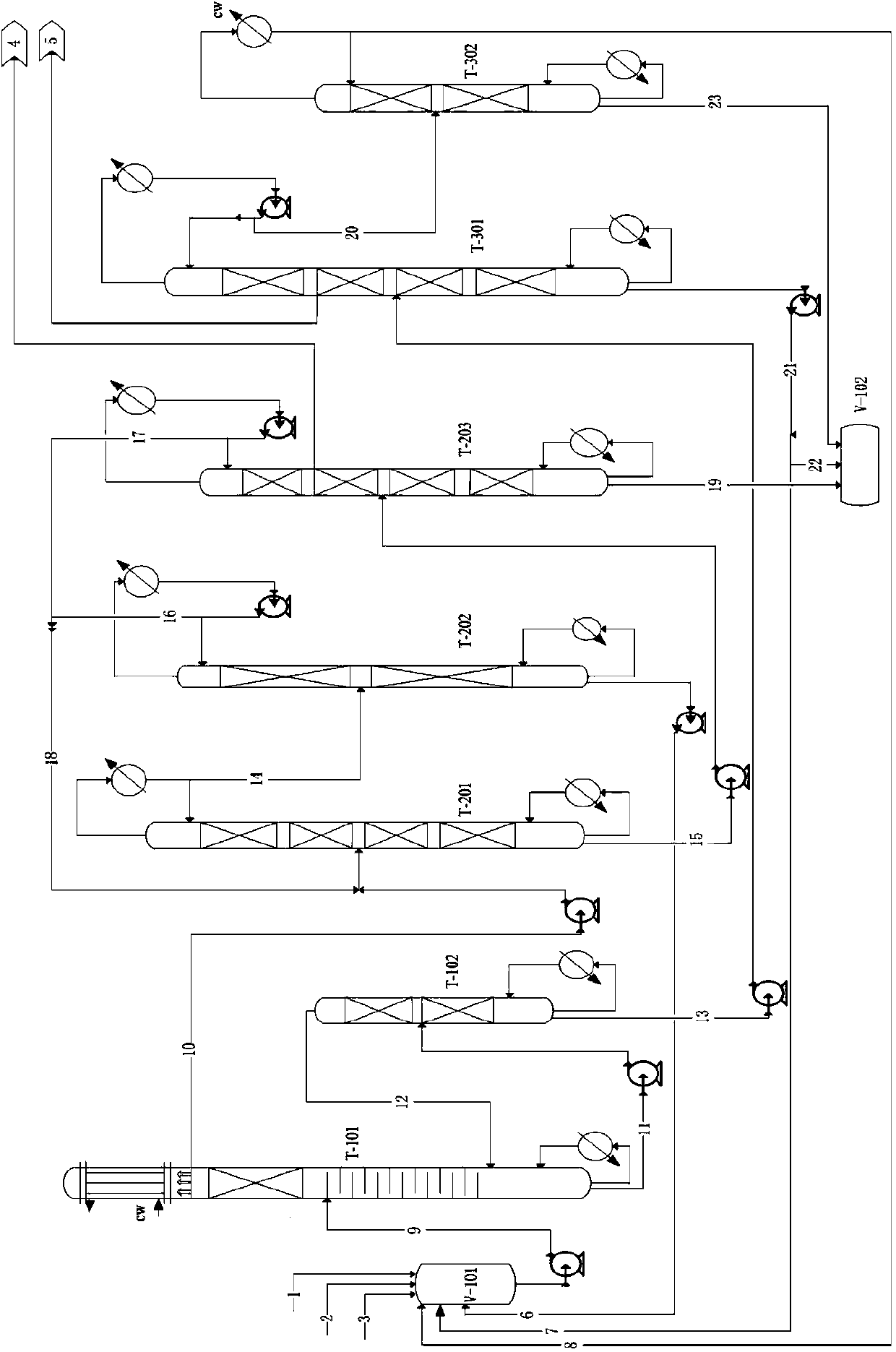
[0062] In the equipment shown in the accompanying drawing, the raw material methanol (982.0g / h), propylene carbonate (1232.0g / h), and ionic liquid catalyst (8.2g / h) are respectively pumped through the raw material pipelines (1, 2, 3). Into batching tank V-101, on the other hand from the methyl alcohol (2921.5g / h) that DMC / methanol atmospheric pressure azeotropic rectification column tank reclaims through pipeline 6 and from the methanol (19.3g / h) that methanol recovery tower top reclaims h) through the material pipeline 8 and also into the batching tank V-101, while the recycling catalyst (70g / h) (catalyst mass content 25-30%) of the propylene glycol product tower T-301 tower bottom material passes through the material pipeline 7 is also poured into the batching tank. After being fully mixed, it is pumped into the middle and upper feed port of the reactive distillation column T-101 through the pipeline 9. Methanol and propylene carbonate react in the reactive distillation colu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com