Method for separating and purifying fusion protein containing chitin binding domain
A technique of combining structural domains and separation and purification, which is applied in the field of fusion proteins and can solve problems such as unsatisfactory adsorption effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Preparation of Partially Deacetylated Chitin Derivative Column Material
[0016] Take 30g of chitin (Aladdin Reagent (Shanghai) Co., Ltd.) in a 1000mL beaker, add 200mL of double distilled water to soak overnight; pour off the double distilled water, add 500mL of 2mol / L HCl to soak for 2h, suction filter, and then wash with water until neutral Add 500mL lmol / L NaOH to soak, and boil the beaker in a 100℃ water bath for 1h, suction filter, and then wash until neutral; add 500mL0.01mol / L NaOH to soak, put the beaker in a 50℃ water bath Boil in water for 1 hour, filter with suction, wash with water until neutral; add 500mL of 0.01mol / LCH 3 COOH, boil the beaker in a 50°C water bath for 0.5h, filter with suction, and wash with water until neutral. Finally, place the treated chitin in a vacuum desiccator for vacuum drying treatment. The temperature of the vacuum desiccator should not exceed 80°C. After complete drying, put the chitin in a mortar for grinding, store...
Embodiment 2
[0017] Example 2 Design and Cloning of a Fusion Protein Containing a Chitin Binding Domain (CBD)
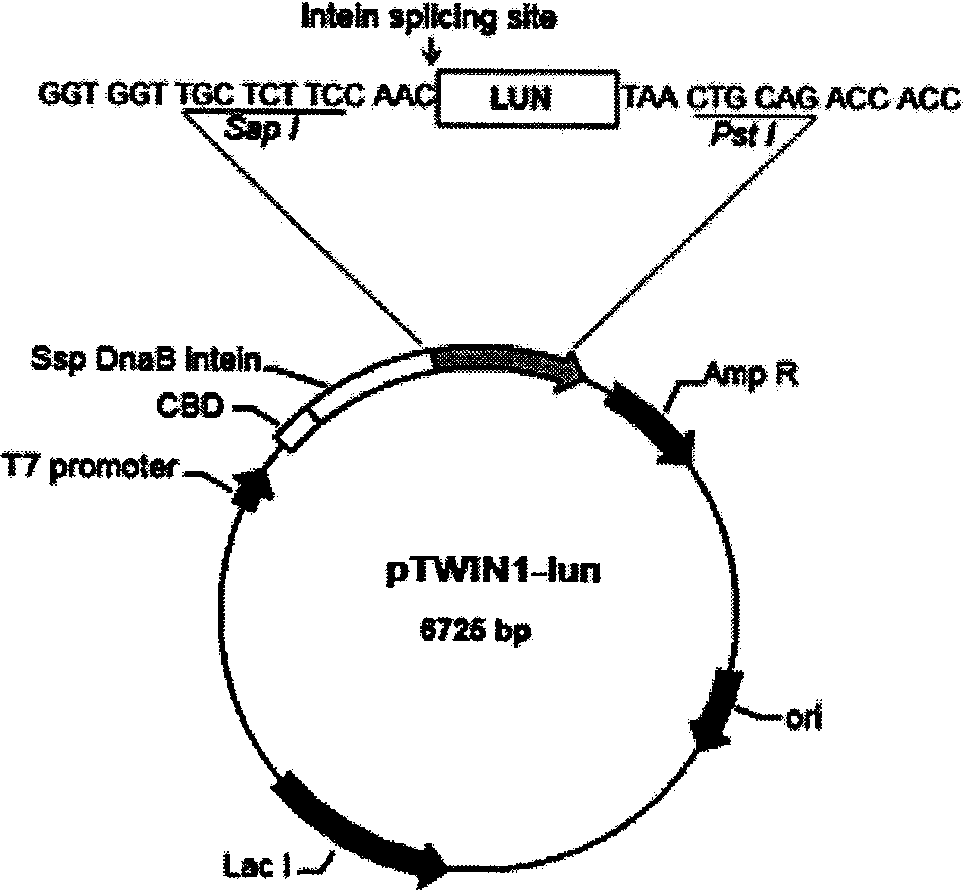
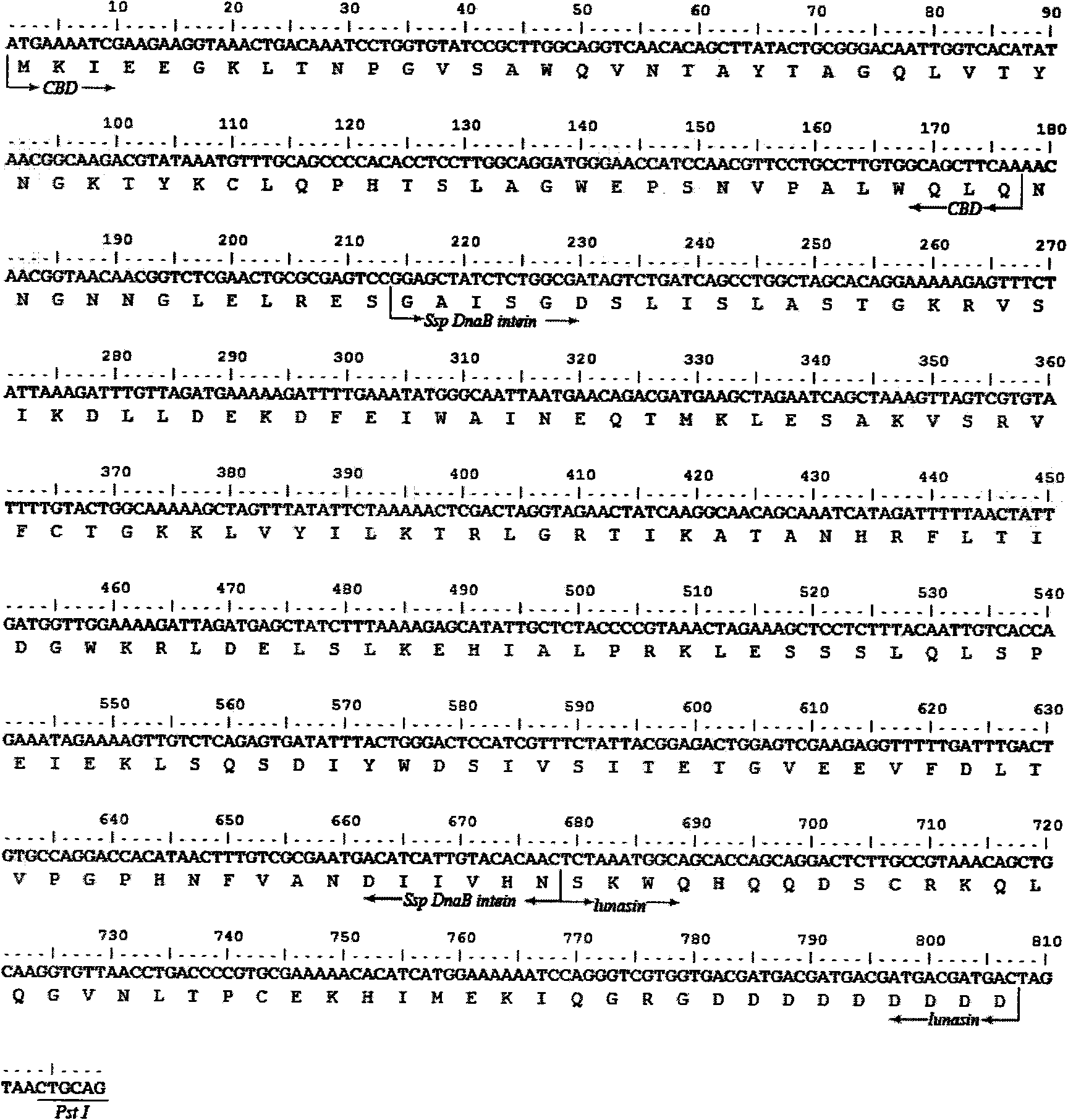
[0018] According to the amino acid sequence of the lunasin polypeptide, the gene of the lunasin polypeptide was designed using OPTIMIZER (http: / / genomes.urv.es / OPTIMIZER) and Gene Designer (http: / / www.DNA20.com) software and codons preferred by Escherichia coli coding sequence, and added Sap I and Pst I restriction sites on both sides. The lunasin polypeptide coding gene sequence containing Sap I and Pst I restriction sites on both sides was obtained by overlap extension PCR method, and TA-cloned into pMD TM In the 19-T Simple Vector (product of TaKaRa Biotech (Dalian) Company), Sap I and Pst I were double digested, and then subcloned into the pTWIN1 vector (product of New England Biolabs Company) to obtain the recombinant plasmid pTWIN1-1un ( figure 1 ), wherein the fusion expression gene sequentially includes chitin binding domain (Chitin Binding Domain, CBD) gene, Ssp DnaB in...
Embodiment 3
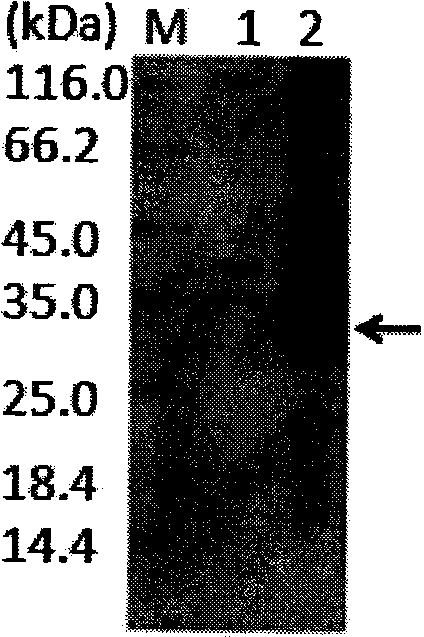
[0019] Example 3 Escherichia coli shake flask expression of CBD-Ssp DnaB intein-lunasin protein and product identification
[0020] Pick a single colony of the positive clone pTWIN1-1un / E.coli BL21(DE3) into 50ml LBA (LB medium with 25μl 20% (w / v) Amp) liquid seed medium, and shake at 220rpm at 37°C for 12h. Seed medium was inoculated in 200ml expression medium (containing 80μl trace element medium, 100μl 20% (w / v) Amp) with 1% (v / v) inoculum amount (1L expression medium contained 25g glycerol; yeast powder 15g ; Peptone 15g; (NH 4 ) 3 PO 4 ·3H 2 O4g; KH 2 PO 4 8g;K 2 HPO 4 ·3H 2 O7g; MgSO 4 ·7H 2 O1g; 1L trace element medium contains HCl5ml; CaCl 2 2H 2 O9g; CoCl 2 ·6H 2 O6g; CuCl 2 2H 2 O2.125g; FeCl 3 ·6H 2 O135g; H 3 BO 3 0.75g; MnCl 2 4H 2 O5g; Na 2 MoO 4 2H 2 O12g; ZnO5g), 37 ° C, 220rpm shaking culture to OD 600nm=0.6-0.7, at this time, 2ml of 20% (w / v) lactose was added to a final concentration of 0.2% (w / v), and the induction expression was c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com