Preparation method of caffeine intermediate N,N-1,3-dimethyl-4,5-diamido urazine
A technology of dimethyl and intermediates, which is applied in the field of synthesis and preparation of organic compounds, and can solve the problems of low yield of dimethyl DAU, poor selectivity and unfavorable Raney nickel catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
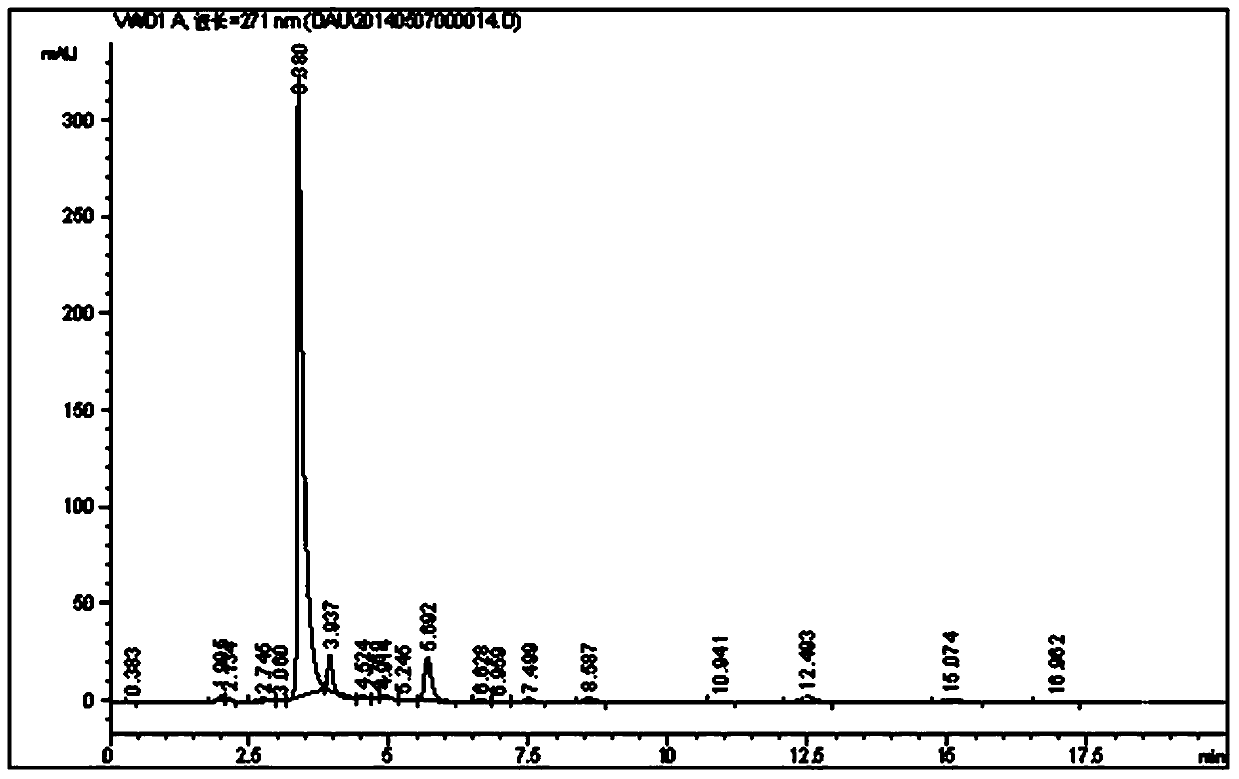
[0021] Example 1 Preparation of caffeine intermediate N,N-1,3-dimethyl-4,5-disemicarbazine according to the present invention
[0022] Weigh 200g of dimethyl NAU dry product, 2400ml of water, 24g of Raney nickel, solid Na 2 CO 3 6g, NaCl1.92g, put into the hydrogenation reduction kettle for replacement, heat the reaction kettle, and start the reaction at 55°C, H 2 The pressure is 0.40MPa, the reaction time is 50min, the yield of dimethyl DAU is 94.5%, and the purity is 95.3%.
Embodiment 2
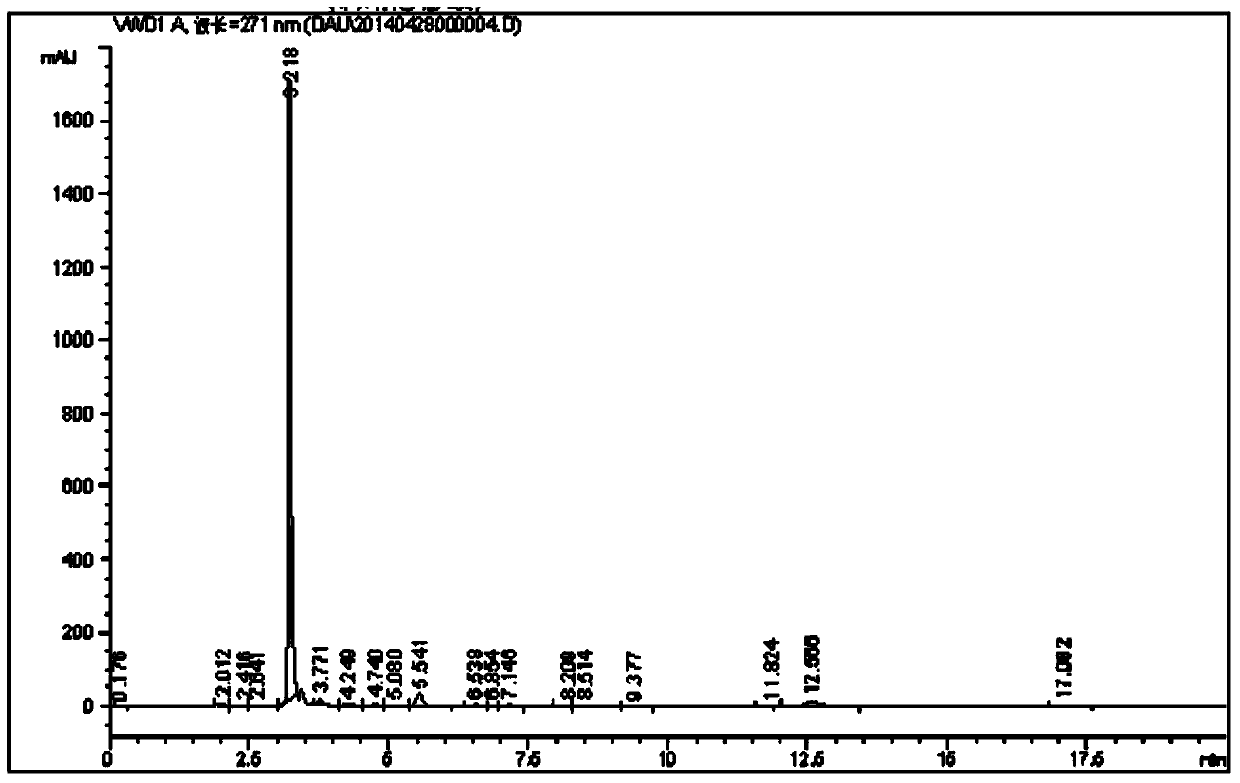
[0023] Example 2 Preparation of caffeine intermediate N,N-1,3-dimethyl-4,5-disemicarbazine according to the present invention
[0024] Weigh 200g of dimethyl NAU dry product, 2400ml of water, 25g of Raney nickel, solid Na 2 CO 3 10g, NaBr2g, put into the hydrogenation reduction kettle for replacement, heat the reaction kettle, start the reaction at 55°C, H 2 The pressure is 0.35MPa, the reaction time is 40min, the yield of dimethyl DAU is 93.2%, and the purity is 93.4%.
Embodiment 3
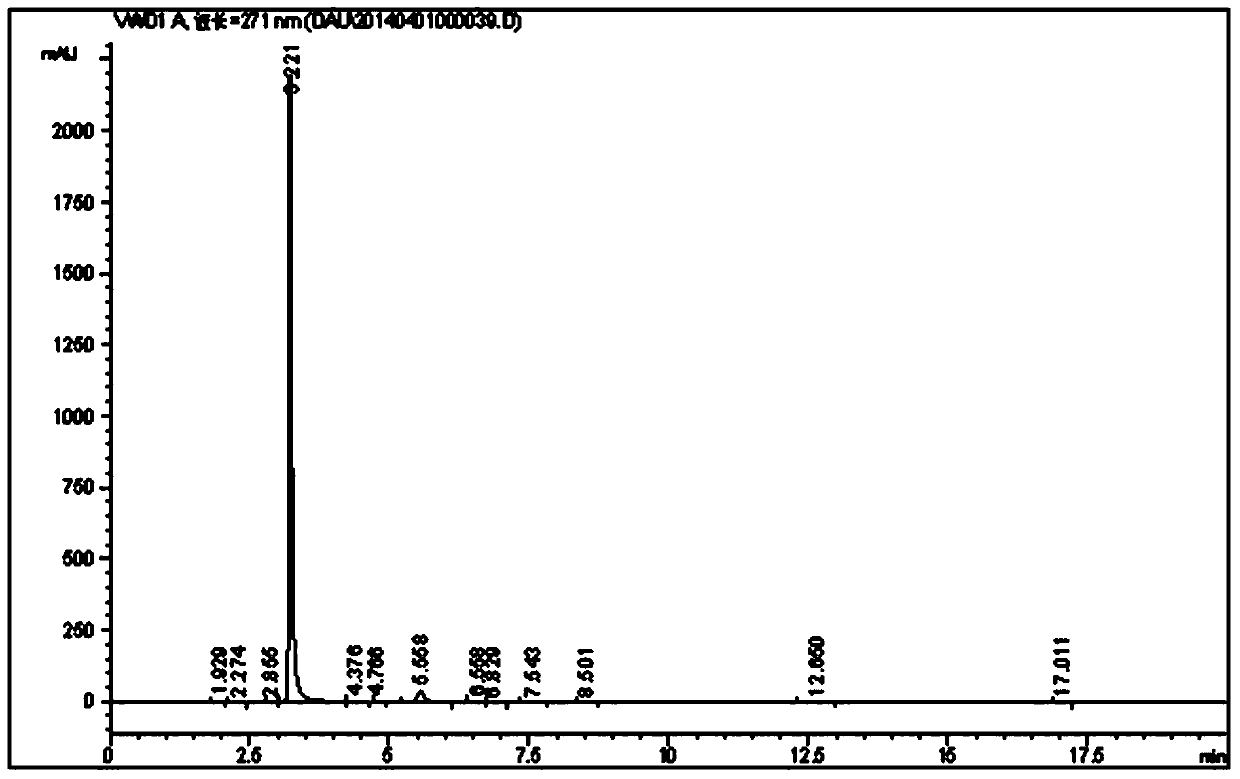
[0025] Example 3 Preparation of caffeine intermediate N,N-1,3-dimethyl-4,5-disemicarbazine according to the present invention
[0026] Weigh 200g of dimethyl NAU dry product, 3000ml of water, 15g of Raney nickel, solid NaHCO 3 35g, KI0.8g, put into the hydrogenation reduction kettle for replacement, heat the reaction kettle, start reaction at 30°C, H 2 The pressure is 0.60MPa, the reaction time is 100min, the yield of dimethyl DAU is 90.9%, and the purity is 92.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com