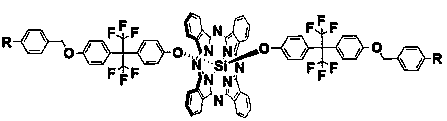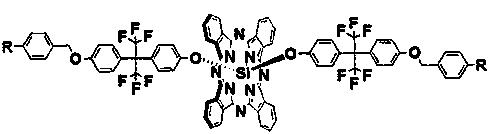Fluoro aryl benzyl ether dendritic (phthalocyanino) silicon complex as well as preparation method and applications thereof
A technology of fluorinated aryl benzyl ether and substituted aryl benzyl ether, which is applied in the field of fluorinated aryl benzyl ether dendritic phthalocyanine silicon complex and its preparation, to achieve the effect of inhibiting self-aggregation behavior and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Dichlorosilicon phthalocyanine (SiPcCl 2 )Synthesis
[0030] Add 1,3-diiminoisoindoline (3.7 g, 25.5 mmol), silicon tetrachloride (4.2 mL) and quinoline (42 mL) into a three-necked flask respectively, stir and reflux at 220 °C for 30 min, When the mixture was cooled to 80 °C, it was poured into methanol (80 mL), filtered while hot, and the filter residue was washed with 35 mL each of toluene, quinoline, methanol and acetone. After drying, 2.2 g of a purple solid was obtained. The yield was 60%.
[0031] 2) 2-(4-hydroxybenzene)-2'-(4-(4-cyano-benzyloxy)hexafluoropropane (abbreviation: G 1 -F-CN-OH) synthesis
[0032] Add p-cyanobenzyl bromide (2.4 g, 12.0 mmol), anhydrous potassium carbonate (2.0 g, 14.5 mmol), 2, 2-bis(4-hydroxyphenyl) hexafluoropropane (4.9 g, 14.4 mmol), acetone (50 mL), stirred vigorously at 55 ℃ and refluxed for 48 h, filtered, collected the filtrate, evaporated the solvent under reduced pressure, and obtained a white crude product. The crud...
Embodiment 2
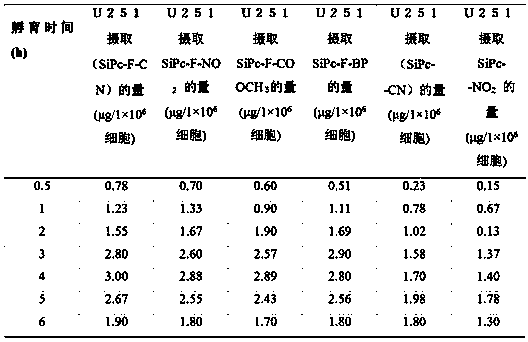
[0049] In Example 1, the process 2) was changed to 4.2 g of p-cyanobenzyl bromide, 9.0 g of 2,2-bis(4-hydroxyphenyl)hexafluoropropane, 50 mL of acetone, and 2 h of reaction time , other reaction conditions were the same, 7.5 g of white powdery solid substance was obtained, and the yield was 73%.
[0050] In embodiment one, process 3) SiPcCl 2 Change to 0.4g, G 1 -F-CN-OH was changed to 0.8g; the temperature was changed to 150°C and other reaction conditions were the same, the blue-green solid was 0.33 g, and the yield was 70.0%.
[0051] In Example 1, the process 4) was changed to 3.8 g of p-nitrobenzyl bromide, 6.0 g of 2,2-bis(4-hydroxyphenyl)hexafluoropropane, 60 mL of acetone, and 3 h of reaction time , other reaction conditions were the same, and 5.5 g of white powdery solid substance was obtained with a yield of 47%.
[0052] In embodiment one, process 5) SiPcCl 2 Change to 0.5g, G 1 -F-CN-OH was changed to 1.0g; the temperature was changed to 150°C, and other react...
Embodiment 3
[0056] In Example 1, the process 2) p-cyanobenzyl bromide was changed to 3.2 g, 2,2-bis(4-hydroxyphenyl)hexafluoropropane was changed to 6.3 g, acetone was changed to 30 mL, and the reaction time was changed to 3 h , other reaction conditions were the same, 4.7 g of white powdery solid substance was obtained, and the yield was 46.0%.
[0057] In embodiment one, process 3) SiPcCl 2 Change to 0.3g, G 1 -F-CN-OH was changed to 0.6g; the temperature was changed to 140°C, and other reaction conditions were the same, the blue-green solid was 0.24 g, and the yield was 60.0%.
[0058] In Example 1, the process 4) was changed to 3.6 g of p-nitrobenzyl bromide, 5.8 g of 2,2-bis(4-hydroxyphenyl)hexafluoropropane, 50 mL of acetone, and 4 h of reaction time , other reaction conditions were the same, and 5.0 g of white powdery solid substance was obtained with a yield of 46.0%.
[0059] In embodiment one, process 5) SiPcCl 2 Change to 0.4g, G 1 -F-CN-OH was changed to 0.7g; the tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com