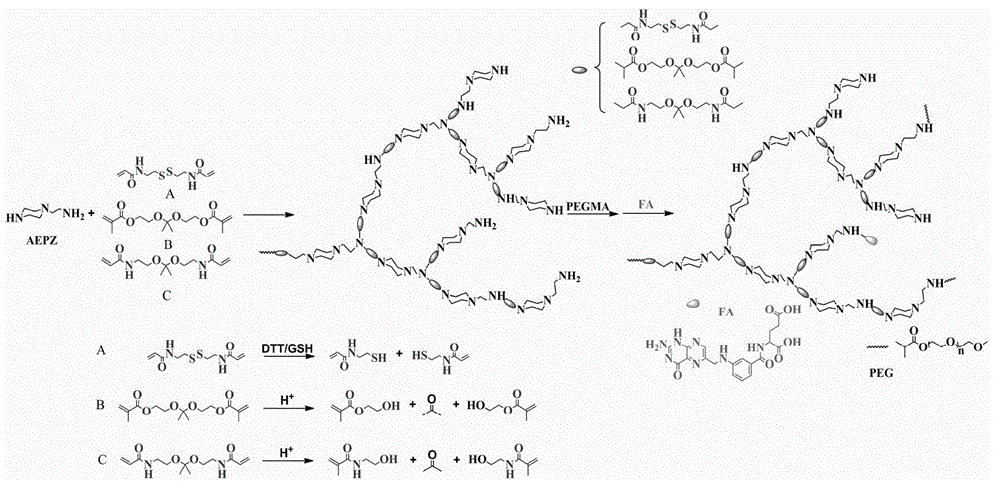
Preparation method and application of degradable hyperbranched polyamidoamine
A technology of polyamidoamine and dimethylformamide, which is applied in the field of preparation of degradable hyperbranched polyamidoamine, can solve the problems that genes and drugs cannot be fully released, achieve good stability and biocompatibility, reduce Effect of interaction and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
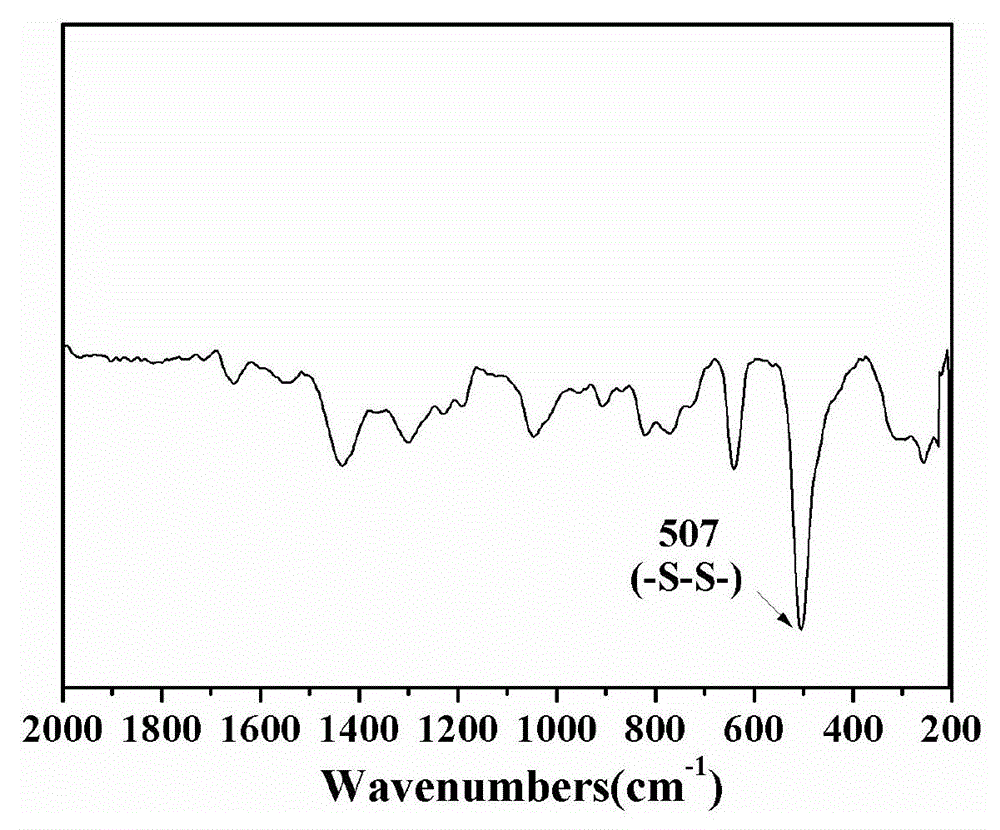
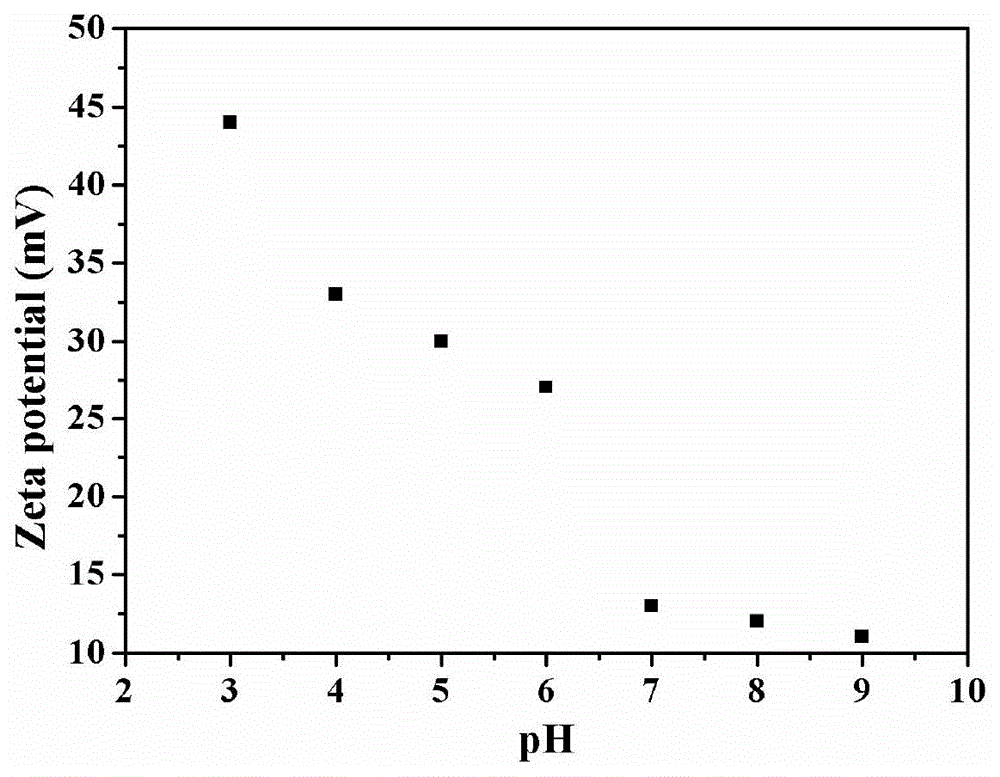
[0024] 3.0 mmol of disulfide bond-containing bifunctional monomer N,N′-bis(acryloyl)cystamine (BAC) and 3.0 mmol of trifunctional monomer 1-(2-aminoethyl)piperazine (AEPZ) were dissolved in methanol Mix in medium temperature, carry out Michael addition polymerization at 50°C under magnetic stirring, add 3 mmol of AEPZ after 5 days and react for 1 day to convert all the double bonds at the end of the polymer into amino groups, and the obtained products are successively precipitated by ether and acetone. The product was purified, and the precipitated product was vacuum-dried at room temperature to obtain a white powder degradable hyperbranched polyamidoamine. Raman, potential test and nuclear magnetic test are carried out to the product obtained, the result is as follows figure 2 and image 3 shown. Among them, such as figure 2 As shown, 507cm -1 is the absorption peak of the disulfide bond, indicating that the disulfide bond can be successfully introduced into the polymer h...
Embodiment 2
[0028] 3.0 mmol of disulfide bond-containing bifunctional monomer N,N′-bis(acryloyl)cystamine (BAC) and 1.50 mmol of trifunctional monomer 1-(2-aminoethyl)piperazine (AEPZ) were dissolved in methanol Mix in medium temperature, carry out Michael addition polymerization at 50°C under magnetic stirring, add 3 mmol of AEPZ after 5 days and react for 2 days to convert all the double bonds at the end of the polymer into amino groups, and the obtained products are successively precipitated by ether and acetone. The product was purified, and the precipitated product was vacuum-dried at room temperature to obtain a white powdery reductively degradable hyperbranched polyamidoamine.
Embodiment 3
[0030] Mix 2.0 mmol of disulfide bond-containing bifunctional monomer N,N′-bis(acryloyl)cystamine (BAC) and 3.0 mmol of trifunctional monomer diethylenetriamine (DETA) in methanol, 50°C, magnetic Carry out Michael addition polymerization under stirring, add 1 mmol of DETA after 5 days and react for 3 days to convert all the double bonds at the end of the polymer into amino groups. The obtained product is purified by ether precipitation, acetone precipitation and other methods successively. Vacuum drying at room temperature gave white powdery reductively degradable hyperbranched polyamidoamine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com