Preparation method of lansoprazole freeze-dried powder injection for injection
A technology for freeze-dried powder injection and lansoprazole, applied in the field of drug production, can solve the problems of inability to meet the compatibility stability, product compatibility stability is not good enough, limited clinical use range and ease of use, etc., and achieve a wide clinical use range. , to avoid the decrease of clarity, good effect of insoluble particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
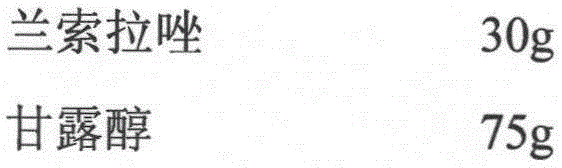
[0015] prescription
[0016]
[0017] Take the prescription amount of lansoprazole and place it in a pressure vessel, add 80% of the total volume of water for injection, under the condition of a relative pressure of 0.06MPa, heat and adjust the water temperature to 105°C, stir for 0.5 hours to obtain a lansoprazole solution, and then Add activated carbon with 2% of the total mass of the solution and stir for 2.5 hours, filter with a 0.45μm microporous membrane under insulation, and adjust the pH to 11.5 by adding 5% sodium hydroxide solution to the filtrate under insulation at 85°C. At room temperature, add the prescribed amount of mannitol and meglumine, and dilute to the full volume with water for injection. Under aseptic conditions, filter with a 0.22μm microporous membrane and fill it in a vial with a rubber stopper. , Put the plate into the freeze dryer, turn on the freeze dryer, start the compressor, cool the slab, set the heat transfer oil inlet temperature to -40 ℃, and k...
Embodiment 2
[0019] prescription
[0020]
[0021] Take the prescription amount of lansoprazole and place it in a pressure vessel, add 90% of the total volume of water for injection, heat and adjust the water temperature to 110°C under the condition of a relative pressure of 0.07MPa, stir for 0.5 hours to obtain a lansoprazole solution, and then add 3% of the total mass of the solution is stirred for 1.5 hours with activated carbon, and filtered with a 0.45μm microporous membrane under the insulation condition. The obtained filtrate is adjusted to 11.0 with 1% sodium hydroxide solution under the insulation condition at 85℃, and the pH is reduced to room temperature. , Add the prescribed amount of mannitol and meglumine, and dilute to the full volume with water for injection, under sterile conditions, filter with a 0.22μm microporous membrane, fill it in a vial, add a rubber stopper to the vial, Put the plate into the freeze dryer, turn on the freeze dryer, start the compressor, cool the slab,...
Embodiment 3
[0023] prescription
[0024]
[0025]
[0026] Take the prescription amount of lansoprazole and place it in a pressure vessel, add 84% of the total volume of water for injection, heat and adjust the water temperature to 108°C under the condition of a relative pressure of 0.065MPa, stir for 0.5 hours to obtain a lansoprazole solution, and then add it The total mass of the solution is 2.6% activated carbon, stirred for 2.0 hours, filtered with a 0.45μm microporous membrane under the insulation condition, and the filtrate obtained is adjusted to pH 11.3 with 0.5% sodium hydroxide solution under the insulation condition at 79°C, and then lowered to room temperature , Add the prescribed amount of mannitol and meglumine, and dilute to the full volume with water for injection, under sterile conditions, filter with a 0.22μm microporous membrane, fill it in a vial, add a rubber stopper to the vial, Put the plate into the freeze dryer, turn on the freeze dryer, start the compressor, cool t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com