A kind of preparation method of quetiapine hemifumarate
A technology of quetiapine fumarate and preparation process, applied in the field of medicine, can solve the problems of low purity of final product, difficult synthesis of intermediates, unstable properties, etc., and achieves simple and easy operation of reaction route, few side reactions, and reduced The effect of separation loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
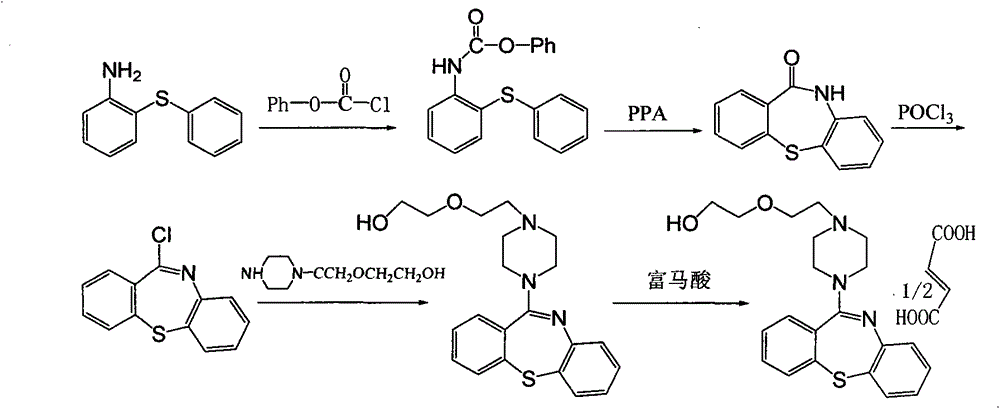
[0019] Embodiment 1: Synthesis of quetiapine hemifumarate through the following steps,
[0020] (1) take o-nitrochlorobenzene and thiophenol as raw materials, carry out etherification reaction in a toluene solvent, and dry to obtain 2-nitrodiphenyl sulfide;
[0021] (2) Ethanol is pumped into the reaction tank, put into 2-nitrodiphenyl sulfide and disposable reduced iron powder for hydrogenation reaction, filter, adjust the pH value of the filtrate with hydrochloric acid, and dry to obtain 2-aminodiphenyl sulfide salt salt;
[0022] (3) Pump chloroform into the reaction tank, add triphosgene, stir, then add 2-aminodiphenyl sulfide hydrochloride, reflux, recover chloroform, without separation and purification, add polyphosphoric acid and p-toluenesulfonic acid, heat , put into purified water, precipitate crystals, and dry to obtain 10-H-dibenzo[b,f][1,4]thiazepin-11-one;
[0023] (4) Chlorinating 10-H-dibenzo[b, f][1,4]thiazepine-11-one and phosphorus oxychloride to obtain 11...
Embodiment 2
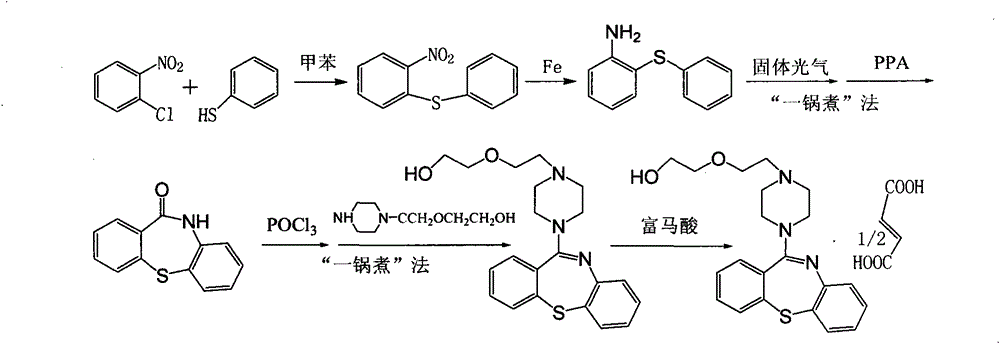
[0024] Embodiment 2: Synthesis of quetiapine hemifumarate through the following steps,
[0025] (1) 180kg toluene, 40kg thiophenol, and 100kg concentration of 50% sodium carbonate solution are sucked into the 500L reaction tank successively, then drop into 50kg o-nitrochlorobenzene, cover the feeding port, reflux reaction at 80°C for 1 hour, and cool to Stand at 40°C for stratification, wash the organic layer twice with water, concentrate under reduced pressure, crystallize, centrifuge, and dry to obtain 79kg of 2-nitrodiphenyl sulfide (yield: 87.4%, HPLC content: 98.5%, melting point: 79-80°C);
[0026] (2) 180kg of ethanol is pumped into the reaction tank, while stirring, drop into 75kg2-nitrodiphenyl sulfide and 20kg disposable reduced iron powder successively, cover the feeding port, open the steam valve, and reflux reaction for 1.5 hours, down to 15°C, filter, adjust the pH value of the filtrate to 1 with hydrochloric acid, precipitate crystals, centrifuge and dry to obt...
Embodiment 3
[0029] Embodiment 3: Synthesis of quetiapine hemifumarate through the following steps,
[0030] (1) 220kg toluene, 50kg thiophenol, and 140kg concentration of 50% sodium carbonate solution are sucked into the 500L reaction tank successively, then drop into 80kg o-nitrochlorobenzene, cover the feeding port, reflux reaction at 90°C for 1.5 hours, and cool to 35°C, let stand to separate layers, wash the organic layer twice with water, concentrate under reduced pressure, crystallize, centrifuge, and dry to obtain 86kg of 2-nitrodiphenyl sulfide (yield: 88.9%, HPLC content: 98.1%, melting point: 80-81°C);
[0031](2) 220kg of ethanol is pumped into the reaction tank, while stirring, drop into 90kg2-nitrodiphenyl sulfide and 40kg disposable reduced iron powder successively, cover the feeding port, open the steam valve, reflux reaction for 2 hours, and drop to 20°C, filter, adjust the pH value of the filtrate to 2 with hydrochloric acid, precipitate crystals, centrifuge and dry to o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com