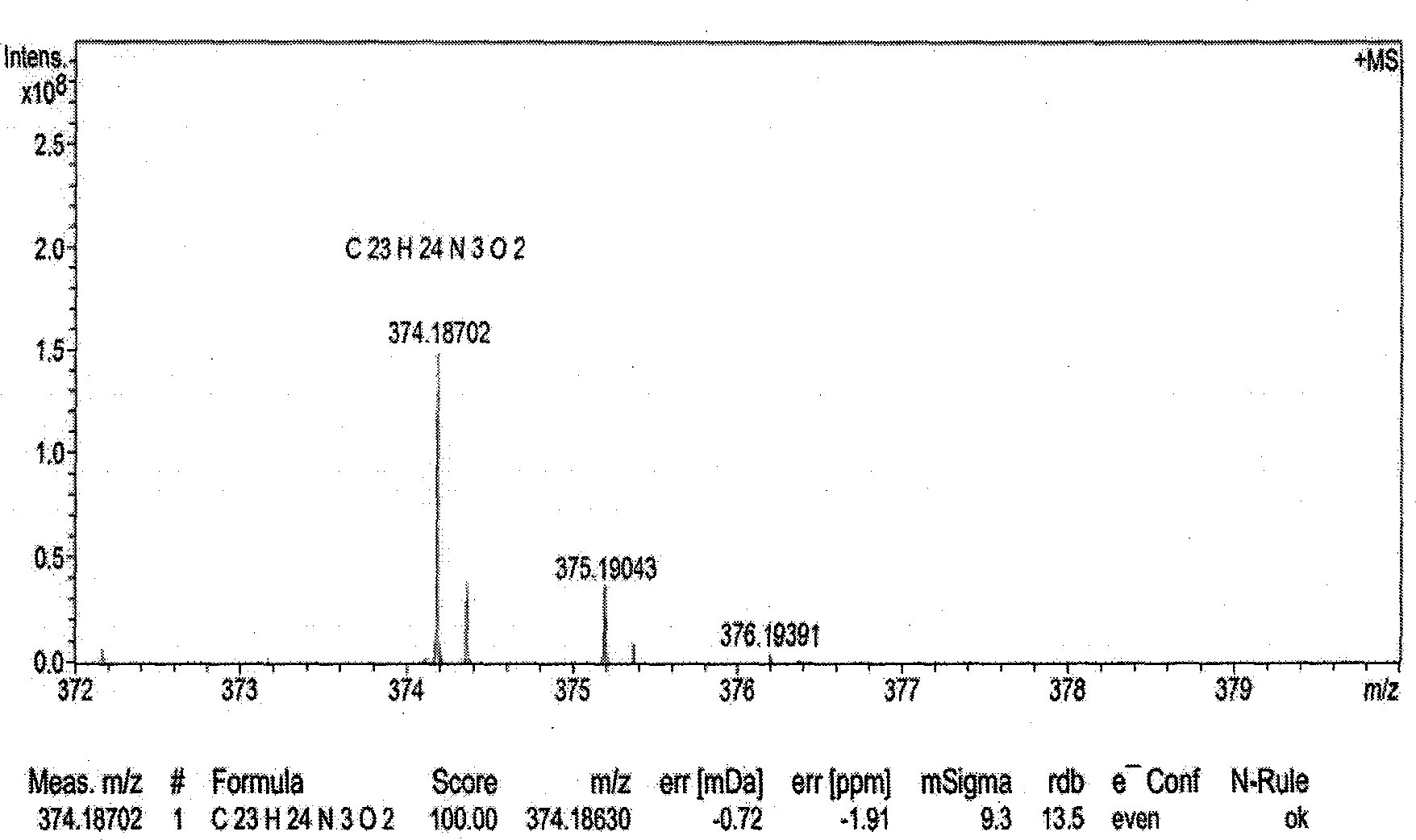
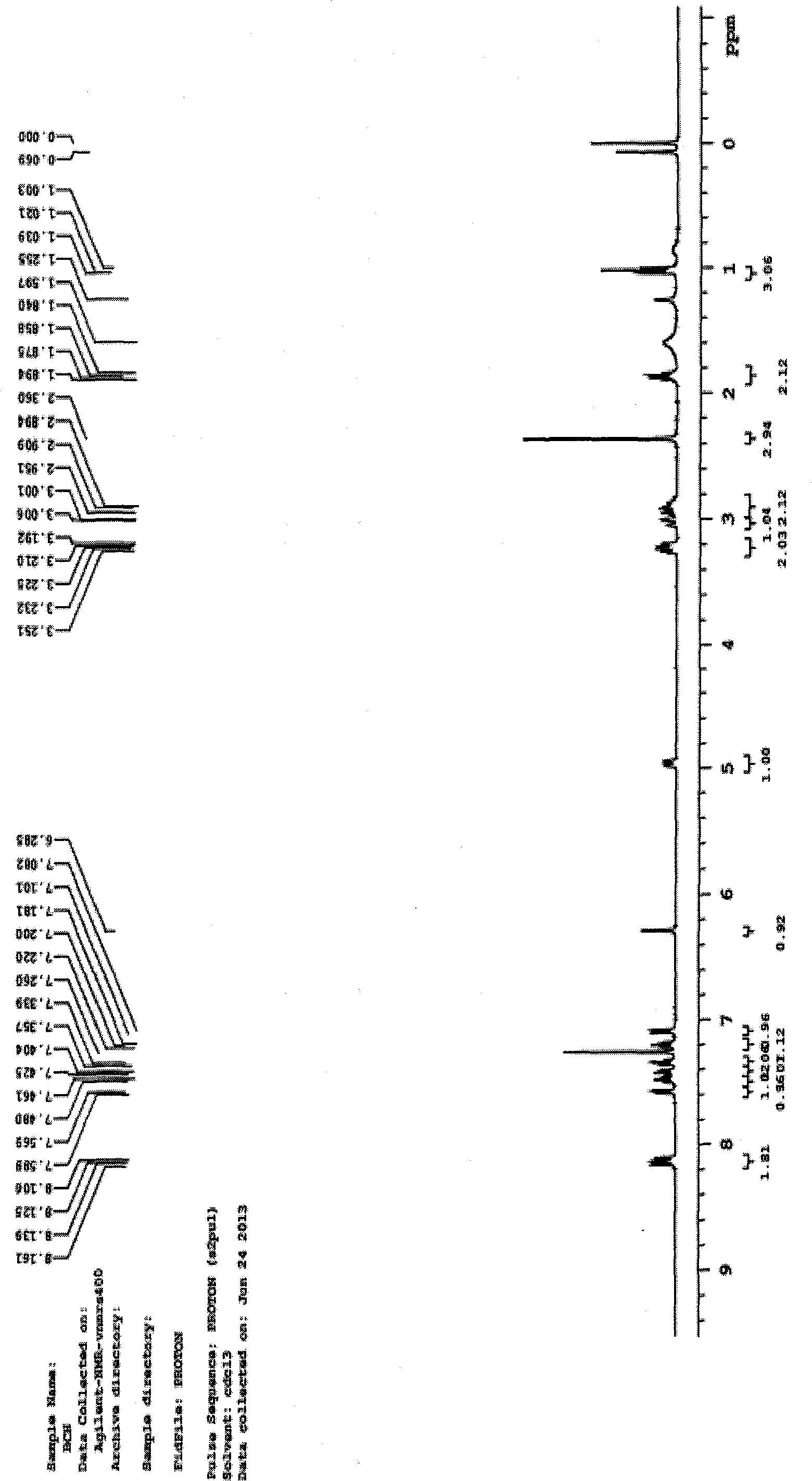
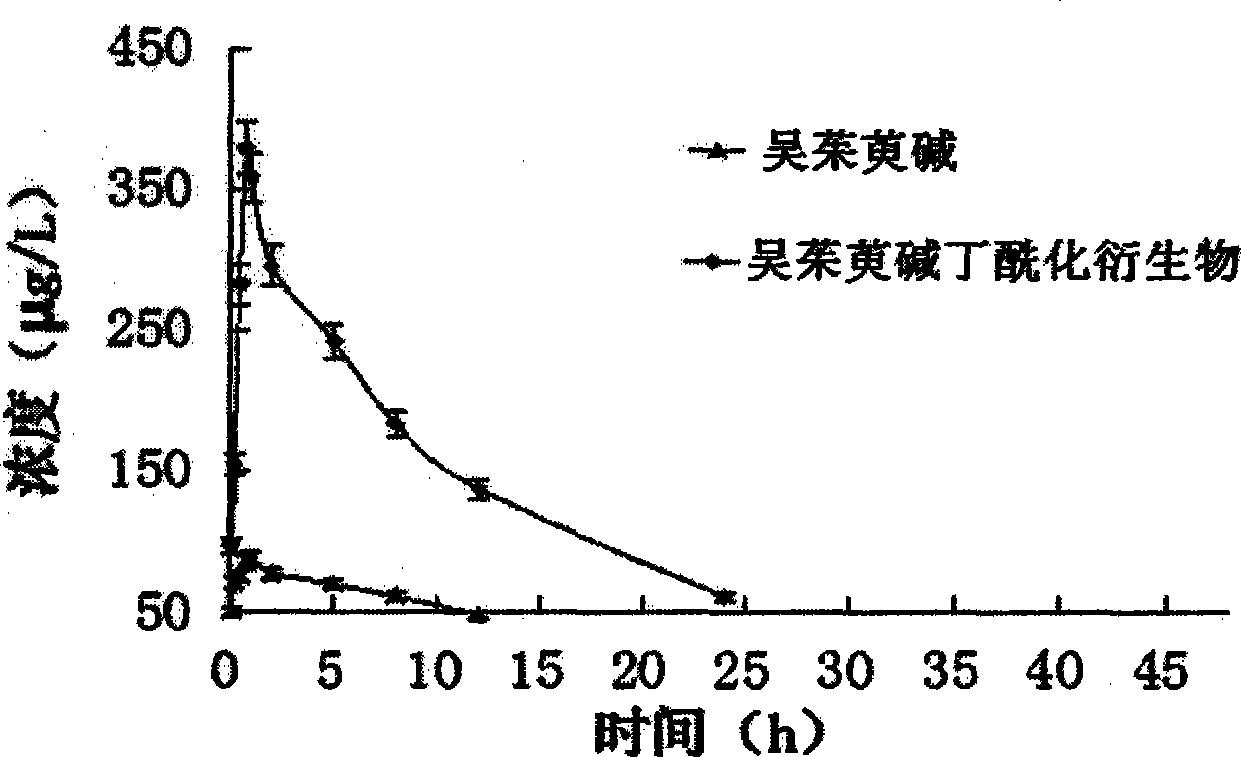
N1-butyrylevodiamine and synthesis method thereof
A technology of butyryl evodiamine and evodiamine, applied in the field of molecular structure and synthesis of N1-butyryl evodiamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Weigh 0.2g of evodiamine (formula II) and place it in a 50ml round-bottomed flask, add 15mL of N,N-dimethylformamide solvent, ultrasonically dissolve, add 0.1323g of sodium hydride (60%) (at this time, evodiamine The molar ratio to sodium hydride is 1:5), after magnetic stirring for 0.5h, add 0.136mL (20°C; density 1.03g / mL) of n-butyryl chloride (formula III) (at this time, evodiamine and n-butyryl chloride The molar ratio is 1:2), the temperature was slowly raised to 70°C, and the reaction was terminated by magnetic stirring for 18 hours; 40 mL of purified water was added to the reaction solution, left to room temperature, and ethyl acetate was added to extract 3 times (40 mL each time). Collect the upper layer extract, add purified water to wash 3 times (each time 50mL), spin dry the solvent, use ethyl acetate:petroleum ether volume ratio of 1:4 solution as eluent, use silica gel column to separate, on silica gel G250 Samples were spotted on thin-layer plates, using ...
Embodiment 2
[0022]Weigh 0.2g of evodiamine (formula II) and place it in a 50ml round-bottomed flask, add 15mL of N,N-dimethylformamide solvent, ultrasonically dissolve, add 0.2630g of sodium hydride (60%) (at this time, evodiamine The molar ratio to sodium hydride is 1:10), after magnetic stirring for 0.5h, add 0.35mL (20°C; density 1.03g / mL) of n-butyryl chloride (formula III) (at this time, evodiamine and n-butyryl chloride The molar ratio is 1:5), the temperature was slowly raised to 80°C, and the reaction was terminated by magnetic stirring for 24 hours; 40 mL of purified water was added to the reaction solution, left to room temperature, and ethyl acetate was added to extract 3 times (40 mL each time). Collect the upper layer extract, add purified water to wash 3 times (50mL each time), remove the solvent by rotary evaporation, use ethyl acetate:petroleum ether with a volume ratio of 1:4 as the eluent, and use a silica gel column to separate. Samples were spotted on G250 thin-layer p...
Embodiment 3
[0024] Weigh 0.2g of evodiamine (formula II) and place it in a 50ml round-bottomed flask, add 15mL of N,N-dimethylformamide solvent, ultrasonically dissolve, add 0.1323g of sodium hydride (60%) (at this time, evodiamine The molar ratio to sodium hydride is 1:5), after magnetic stirring for 0.5h, add 0.401mL (20°C; density 1.03g / mL) of n-butyryl chloride (formula III) (at this time, evodiamine and n-butyryl chloride The molar ratio is 1:6), the temperature was slowly raised to 70°C, and the reaction was terminated by magnetic stirring for 18 hours; 40 mL of purified water was added to the reaction solution, left to room temperature, and ethyl acetate was added to extract 3 times (40 mL each time). Collect the upper layer extract, add purified water to wash 3 times (each time 50mL), spin dry the solvent, use ethyl acetate:petroleum ether volume ratio of 1:4 solution as eluent, use silica gel column to separate, on silica gel G250 Samples were spotted on thin-layer plates, using ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com