Exenatide long-acting microsphere preparation and preparation method thereof
A technology of exenatide and microspheres, applied in the field of medicine, can solve problems such as unsatisfactory research results, and achieve the effects of excellent sustained release performance, low burst release rate and low bacterial infection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Different organic solvents of embodiment 1 are used to prepare exenatide PLGA microspheres
[0030] In this example, dimethyl carbonate (DMC), dichloromethane (DCM), and ethyl acetate (Et-Ac) were selected as organic solvents to prepare three groups of microsphere preparations. The formulations of the three groups of microsphere preparations are shown in Table 1. The formula of each microsphere preparation is 10wt% exenatide and 90wt% polyester.
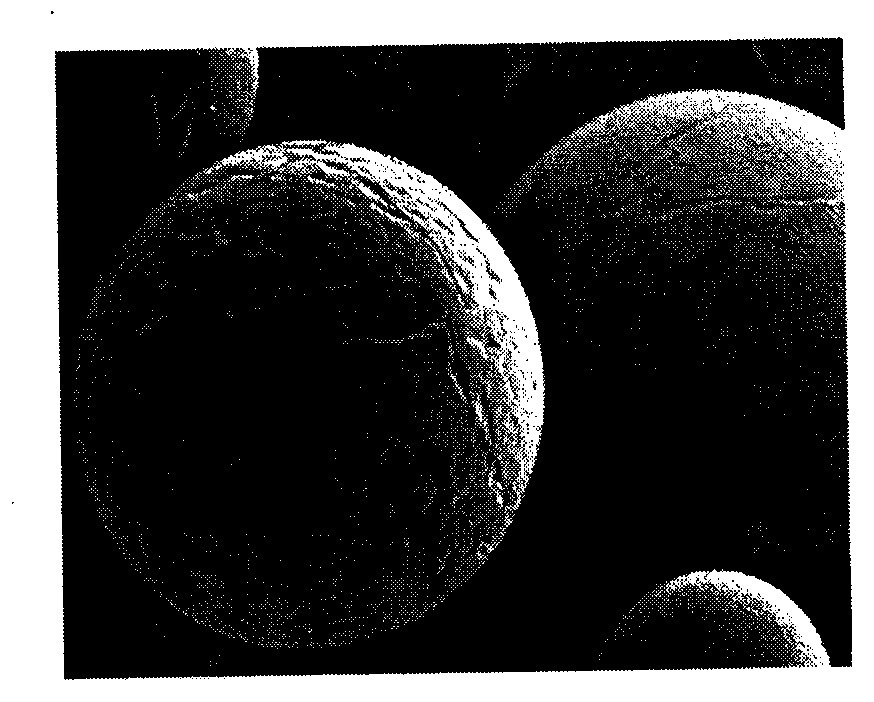
[0031] The type of solvent has a significant impact on the morphology of the product. In this example, exenatide PLGA microspheres were prepared by different organic solvents. The preparation method is as follows (see figure 1 ):
[0032] (1) Weigh 0.1 g of exenatide (1) and 0.9 g of PLGA at the end of the ester group (molecular weight is 40,000, LA / GA=50 / 50);
[0033] (2) Put the PLGA (molecular weight is 40,000, LA / GA=50 / 50) at the end of the ester group in the step (1) into the organic solvent to completely dissolve to ...
Embodiment 2
[0039] Example 2 Preparation of exenatide microspheres of PLGA with different types of ester groups
[0040] This example prepares group 1: the exenatide microsphere preparation of the PLGA (molecular weight is 40,000, LA / GA=50 / 50) of ester group end, group 2: the PLGA (molecular weight is 45,000, LA / GA=50 / 50) of ester group end 75 / 25) exenatide microsphere preparation, the organic solvent used in this embodiment is a mixed solution of dichloromethane (DCM) and dimethyl carbonate (DMC), wherein the exenatide microsphere formulation of Group 1 is 32wt% exenatide and 68wt% polyesters, specifically 0.45g exenatide and 0.97g ester-terminated PLGA (molecular weight 40,000, LA / GA=50 / 50), the preparation method is:
[0041] (1) Weigh 0.45g exenatide and 0.97g ester-terminal PLGA (molecular weight is 40,000, LA / GA=50 / 50) in proportion;
[0042] (2) Put the PLGA (molecular weight is 40,000, LA / GA=50 / 50) in step (1) into dichloromethane (DCM) and dimethyl carbonate (volume ratio is 1: ...
Embodiment 3
[0055] Example 3 Preparation of Exenatide Microspheres with Different Drug Loading Amounts
[0056] In this implementation, three groups of exenatide microspheres with drug loadings of 15.6%, 24.5% and 33.5% were prepared respectively, and the specific formulations are shown in Table 3:
[0057] Table 3: the formula of this embodiment
[0058]
[0059] The concrete preparation method of A preparation is as follows:
[0060] (1) Weigh 0.28g exenatide and 1.5g ester-terminated PLGA (molecular weight is 40,000, LA / GA=50 / 50);
[0061] (2) 1.5g PLGA (molecular weight is 40,000, LA / GA=50 / 50) in the step (1) is dropped into the mixed solution of dichloromethane and dimethyl carbonate and completely dissolved to obtain a clear solution, and then poured into the clear solution Add exenatide and mix and stir evenly to obtain a homogeneous oil solution with a viscosity of 1.76mPa.s;
[0062] (3) The homogeneous oil solution in step (2) is fed into the ultrafine particle preparation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com