Monellin protein mutant and preparation method thereof
A protein mutant and protein technology, applied in the field of protein mutants, can solve the problems such as the inability of large-scale fermentation production of monellin protein, hidden dangers of fermented products, production and transportation restrictions, etc., to save the cost of later purification, reduce the probability of contamination, The effect of increasing the sweetness of the protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of single-chain monellin mutant gene protein
[0025] (1) Construction of wild-type single-chain monellin protein carrier
[0026] The wild-type single-stranded monellin whole gene was synthesized by Zhongmei Taihe Biotechnology Co., Ltd., and two restriction endonuclease sites of XhoI and NotI were primers at both ends, and the target gene fragment and PGAPZαA empty plasmid were digested with T4 DNA ligase Ligate overnight at 37°C, transform into DH5α Escherichia coli competent cells, spread on LLB solid medium containing 25 μg / ml Zeocin, culture overnight to pick positive transformants, use plasmid extraction kit to extract plasmids for sequencing verification, and successfully construct wild type The monellin protein carrier PGAPZαA-SCM.
[0027] (2) Site-directed mutation of wild-type single-chain monellin protein
[0028] Use XhoI and NotI two restriction endonucleases to cut out the target gene fragment, and the reaction system is as follows:
[002...
Embodiment 2
[0043] Determination of thermal stability and sweetness threshold of single-chain monellin mutant proteins
[0044] (1) Identification of protein thermal stability
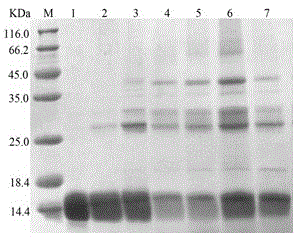
[0045] The same batch of purified proteins were treated at 65°C, 70°C, 75°C, 80°C, and 85°C for 2h, 4h, 6h, 8h, and 10h respectively. The treated samples were detected by SDS-PAGE, and the strips of the gel were observed. With brightness, initially determine the denaturation temperature and maximum tolerance temperature of the protein. It has been detected that the mutant protein E2N still exists after being treated at 75°C for 6 hours and at 80°C for 2 hours (the attached figure figure 1 ), the thermal stability of the wild-type single-chain monellin protein was increased by 10°C.
[0046] (2) Determination of protein sweetness threshold
[0047] In the control group, 10000 μg / ml sucrose was used to dilute the protein into a gradient from 0.25 to 150 μg / ml. Let 10 healthy adults, 5 males and 5 females taste ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com