Recombinant plasmid efficiently expressing xylanase and construction method for recombination Pichia pastoris
A Pichia pastoris and xylanase technology, which is applied in the field of expressing high-efficiency xylanase recombinant plasmids and the construction of recombinant Pichia pastoris, can solve the problems of rapid development and achieve high activity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
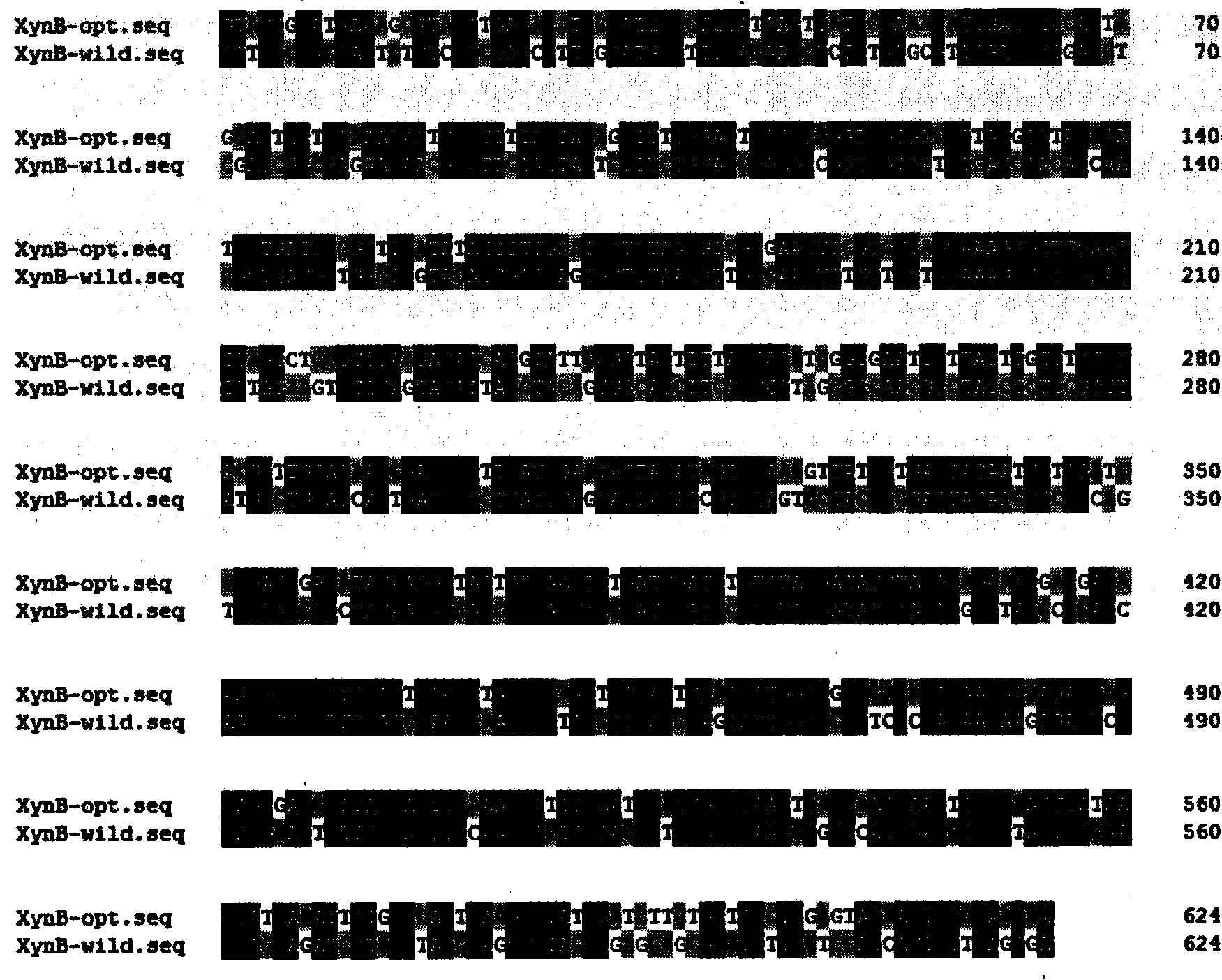
[0029] Optimization of codons in xylanase gene
[0030] According to the codon preference of Pichia pastoris, the codon optimization of xylanase gene was completed with DNA works software. The optimization result is as figure 1 shown.
Embodiment 2
[0032] Construction of single-copy expression vector pPICZαA-XynB-opt
[0033] The optimized xylanase gene sequence XynB-optimized (XynB-opt) was synthesized by Beijing Qingke Xinye Biotechnology Co., Ltd. Restriction sites were designed at both ends of the gene, EcoR I and Xba I respectively, and the gene was connected to the pPICZαA vector. The successfully constructed recombinant expression vector pPICZαA-XynB-opt was transformed into Escherichia coli competent E.coil top10, and positive clones were screened on LB low-salt culture plates.
Embodiment 3
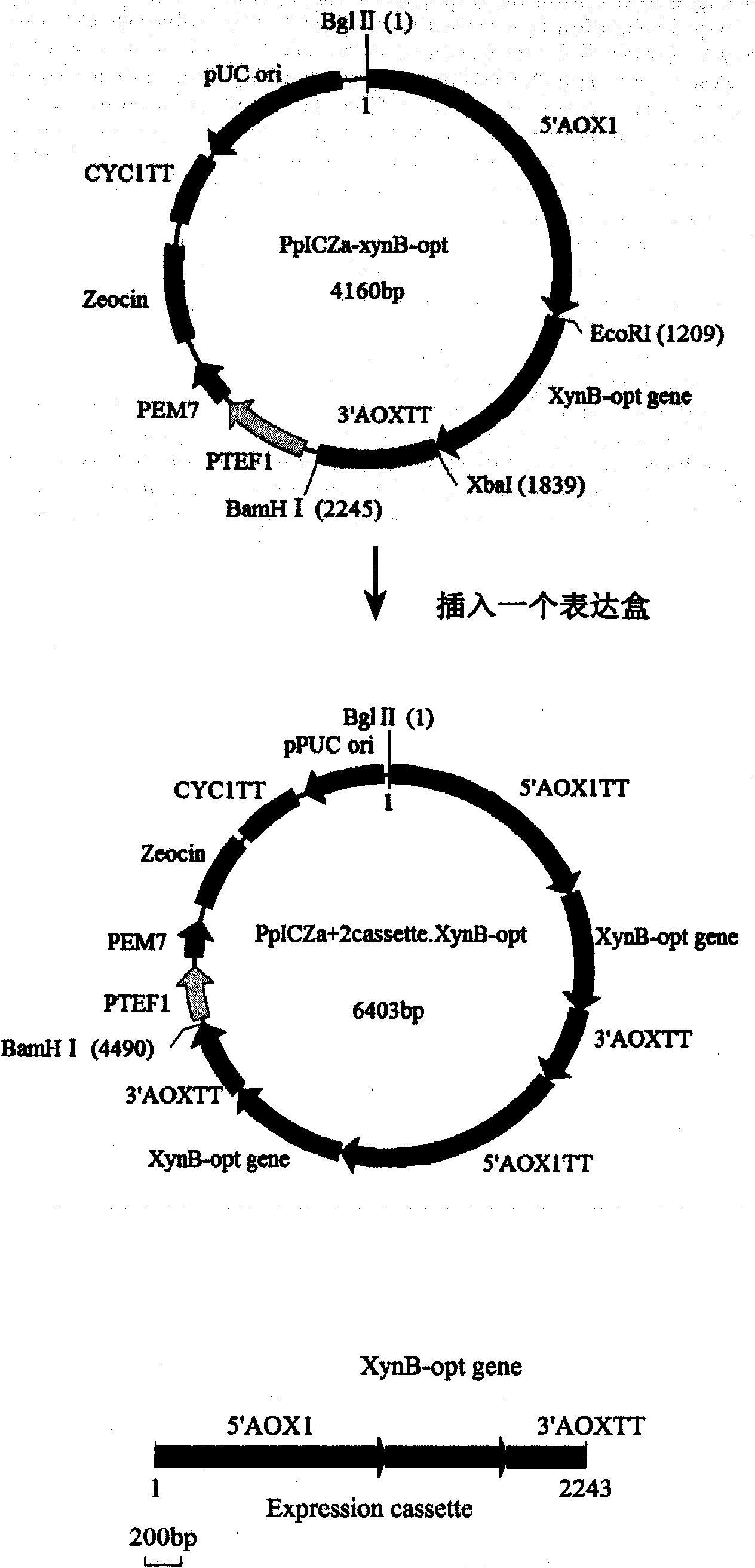
[0035] pPICZαA-(XynB-opt) 2 Construction of double-copy expression vector
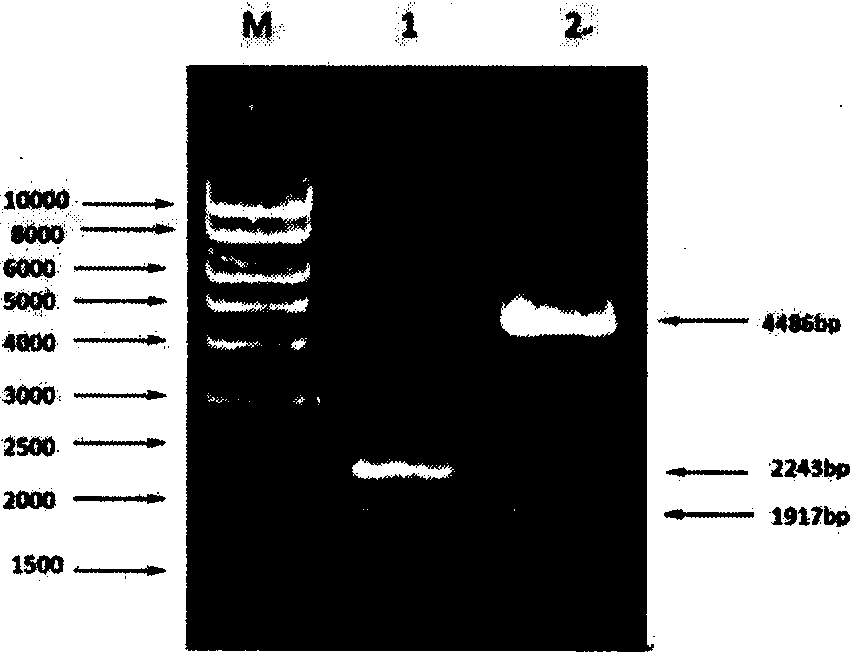
[0036] Double copies of XynB-opt were constructed by in vitro tandem method. The pPICZαA-XynB-opt recombinant plasmid stored in our laboratory was single-digested with Bgl II, double-digested with BamH I and Bgl II, and kept in a water bath at 37°C overnight. After the xynB gene fragment was recovered by 0.8% agarose gel electrophoresis, the double-digested product was ligated with the purified single-digested product with T4 DNA ligase overnight at 16°C. Build process like figure 2 shown.
[0037] Transform the ligation product into E. coil Top10 competent cells, incubate at 37°C for 1 h, spread the bacterial solution on LB solid medium containing 25 μL / 100mL Zeocin, and incubate overnight at 37°C. After visible colonies grow, use Cracking to quickly identify recombinant plasmids, and initially screen out double-copy recombinant plasmids. Pick double-copy recombinant plasmid colonies, inoculate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com