Manufacturing method of bis(2-ethylhexyl) terephthalate
A technology of di- and ethylhexyl terephthalate, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, to achieve the effects of reducing reaction time, improving filtration rate, and increasing reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
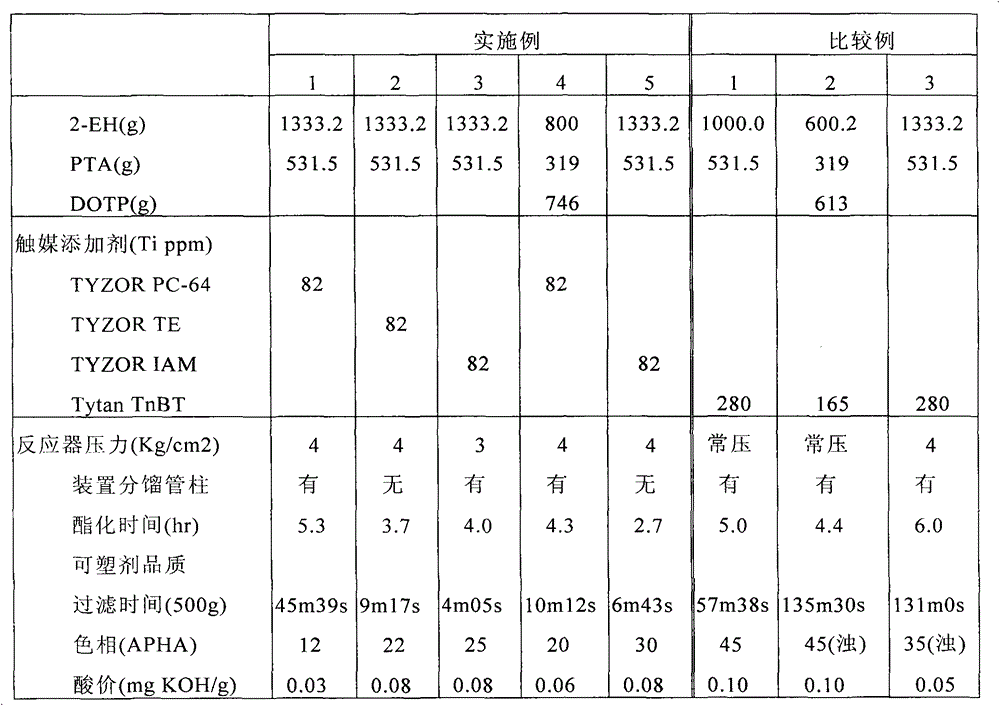
[0028]In a 3L autoclave equipped with stirrer, thermometer, back pressure valve manometer, oil-water separator, return line, nitrogen flow controller, condenser and 30cm filling fractionation column, 1333.2g (10.24mole) Isooctyl alcohol (also known as 2-ethylhexanol, 2-EH), 531.5g (3.20mole) of pure terephthalic acid (PTA), 3.07g (relative to the total weight of the reaction system alcohol and acid reactants TyzorPC-64 (phosphate titanate chelate, Ti=5.0%, purchased from DORF KETAL CO.) catalyst equivalent to Ti=82ppm) was put into the reaction kettle. The reaction kettle was fully stirred under nitrogen flow and pressurized to a gauge pressure of 4.0kg / cm 2 , make the temperature rise to 232°C within 1 hour (starting esterification distillation temperature), the water and alcohol mixture brought out by the nitrogen flow begins to condense in the oil-water separator after filling the fractionation column and the condenser, and the upper alcohol mixture is refluxed The pipe is...
Embodiment 2
[0030] Use the same reactor and reaction compound as in Example 1, but replace the packed fractionation column with a 30cm single tube, and the catalyst system uses Tyzor TE (two (triethanolamine) diisopropyl titanate chelate), Ti = 8.2%, purchased from DORF KETAL CO. product). 1333.2g (10.24mole) of 2-EH, 531.5g (3.20mole) of PTA and 1.87g of Tyzor TE (Ti=82ppm) were put into the reactor. The reaction kettle is fully stirred under nitrogen flow and pressurized to a gauge pressure of .4.0kg / cm 2 , and heated up to 233°C in 1 hour, the water and alcohol mixture brought out by the nitrogen flow began to pass through the single tube (meaning the column without filler) and the condenser, and then condensed in the oil-water separator, and the upper alcohol mixture was led by the return pipe Return to the reactor to ensure that the reaction 2-EH / PTA is maintained at a fixed molar ratio of 3.2 / 1, and the water in the lower layer is continuously distilled out of the oil-water separat...
Embodiment 3
[0032] A reactor was used as in Implementation 1, but Tyzor IAM (trialkoxyphosphate butyl titanate dimer, Ti=8.8%, purchased from DORF KETAL CO.) was used as catalyst. 1333.2g (10.24mole) of 2-EH, 531.5g (3.20mole) of PTA and 1.68g of Tyzor IAM (Ti=82ppm) were put into the reactor. The reaction kettle was fully stirred under nitrogen flow and pressurized to a gauge pressure of 3.0kg / cm 2 , and heated up to 227°C within 1 hour, the water and alcohol mixture brought out by the nitrogen flow began to condense in the oil-water separator after filling the fractionation column and condenser, and the upper alcohol mixture was led back to the reactor through the return pipe to ensure the reaction 2 -EH / PTA is maintained at a fixed molar ratio of 3.2 / 1, and the water in the lower layer is continuously distilled out of the oil-water separator and taken out for metering. The reaction continues to heat up to 250°C, and after 3.7 hours, remove water from the separator and measure 116 mill...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com