Culture method for improving oxidative metabolic capability of chicken skeletal muscle cells
A technology for skeletal muscle cells and metabolic capacity, which is applied in the field of cultivating the oxidative metabolic capacity of chicken skeletal muscle cells. It can solve the problems of strong randomness of muscle fiber types and low oxidative metabolic capacity, so as to improve oxidative metabolic capacity and inhibit myoblasts. Effects of apoptosis and promotion of myogenic differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
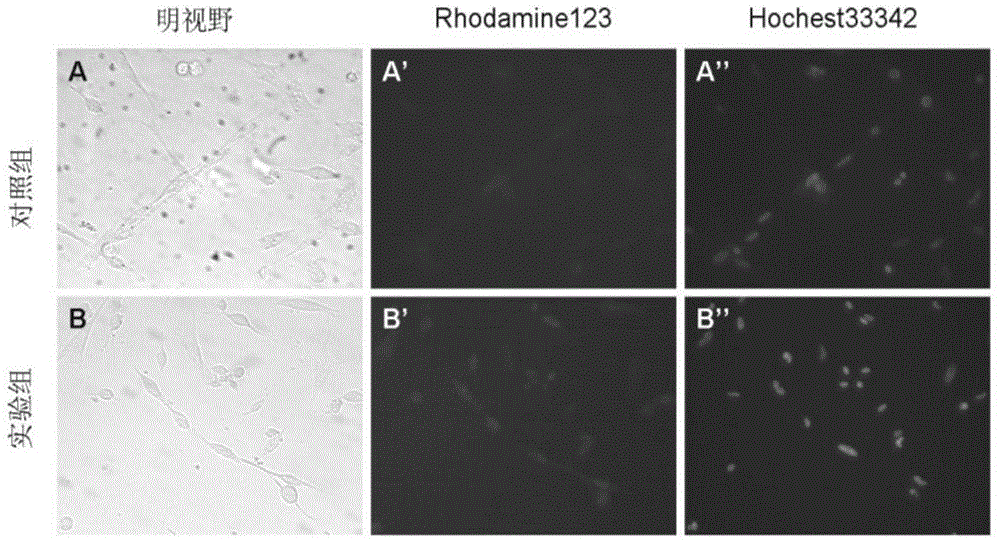
[0038] Embodiment 1 Isolation, purification and proliferation of chicken skeletal muscle myoblasts
[0039] 1. Isolation and purification of chicken skeletal muscle myoblasts
[0040] Fresh eggs were hatched in an incubator at 38°C and 63% humidity for 11 days. After the eggshells are sterilized by 70% ethanol, the eggshells are broken and the chicken embryos are taken out and placed in a petri dish (if the chicken embryos are dead, discard them to prevent contamination). Use tweezers to remove the breast skin of the chicken embryo, separate the pectoralis major muscle, and remove ligaments, connective tissue and blood vessels under a dissecting microscope. Wash the pectoralis tissue 3 times with Hank's solution, shred it thoroughly, add 0.1% collagenase I and incubate at 37°C for 30 minutes, blowing several times during the period. Centrifuge, add Hank's to blow the cells, and filter the cells with a 200-mesh stainless steel filter to obtain a single-cell suspension.
[00...
Embodiment 2
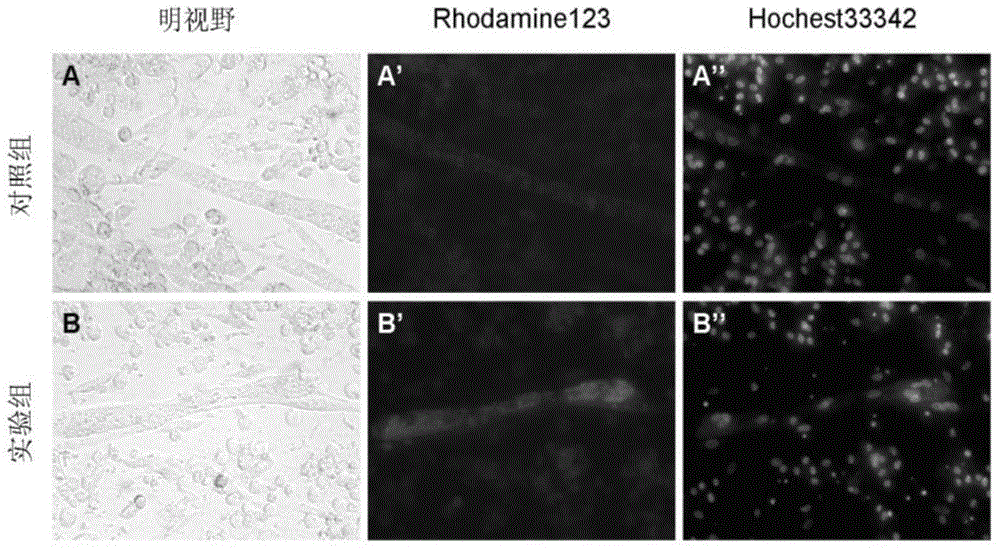
[0044] Embodiment 2 Isolation and cultivation of chicken skeletal muscle myogenic fibroblasts
[0045] Fresh eggs were hatched in an incubator at 38°C and 63% humidity for 11 days. After the eggshells are sterilized by 70% ethanol, the eggshells are broken and the chicken embryos are taken out and placed in a petri dish (if the chicken embryos are dead, discard them to prevent contamination). Use tweezers to remove the breast skin of the chicken embryo, separate the pectoralis major muscle, and remove ligaments, connective tissue and blood vessels under a dissecting microscope. Wash the pectoralis tissue 3 times with Hank's solution, shred it thoroughly, add 0.1% collagenase I and incubate at 37°C for 30 minutes, blowing several times during the period. Centrifuge, add Hank's to blow the cells, and filter the cells with a 200-mesh stainless steel filter to obtain a single-cell suspension. The cells were seeded on a culture dish, and after culturing at 37°C for 2 hours, the a...
Embodiment 3
[0047] The differentiation culture of embodiment 3 chicken skeletal muscle myoblasts
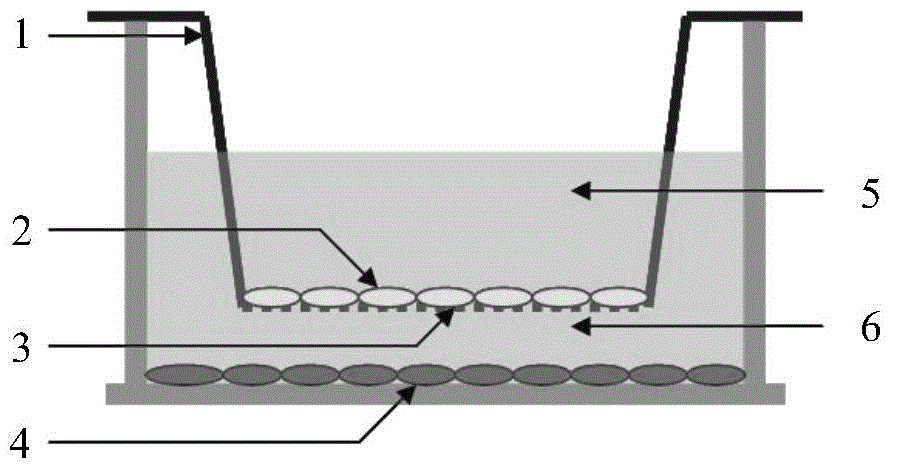
[0048] In order to avoid physical contact between myoblasts and fibroblasts during the culture process, and at the same time exert the myogenic effect of myogenic fibroblasts, this example uses a Transwell cell culture system with a 1um pore size PET membrane. The operation steps of myoblast differentiation culture include: using 2000 cells / cm 2 The density of myogenic fibroblasts (Example 2) through 3-5 passages was inoculated in a Transwell culture dish, and the medium was high-sugar DMEM containing 10% FBS, 100U / ml penicillin, and 100ug / ml streptomycin After culturing at 37°C for 1 day, remove the above-mentioned medium and wash 3 times with PBS; transfer the Transwell Insert (step 2 of embodiment 1) inoculated with chicken skeletal muscle myoblasts into the above-mentioned Transwell culture dish, and along the inner wall of the culture dish Add slowly containing 60umol / L H 2 o 2 Myoge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com