Preparation method of novel anti-infective drug
An anti-infection and drug technology, applied in the field of medicine, can solve the problems of easy residue, increased impurity content, and high product impurity content, and achieve the effects of reducing product impurity content, reducing degradation impurities, and improving product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
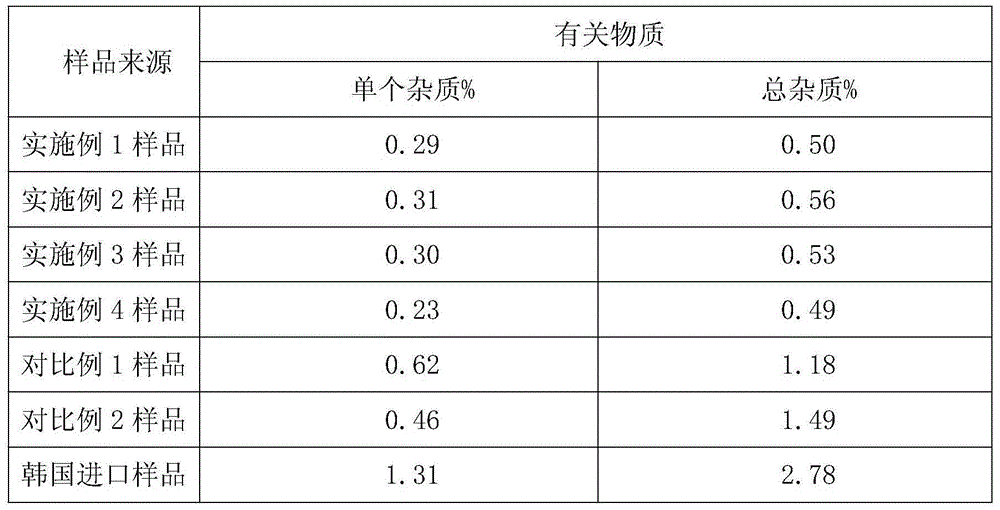
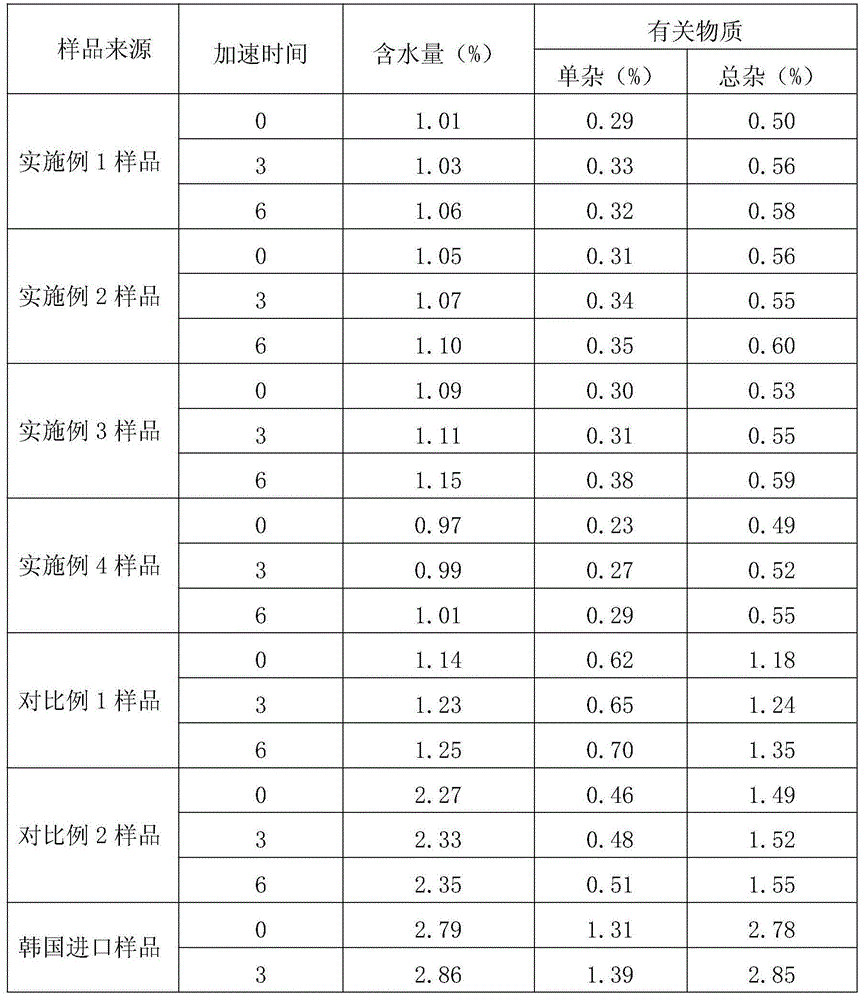
Embodiment 1
[0030] A kind of preparation method of novel anti-infective drug cefazedone sodium, the steps are as follows:
[0031] (1) Salt formation: Aseptically treat the reaction kettle, filter and utensils used, add 1096.9g (2mol) cefazinone, 164g (2mol) sodium acetate and 4.39L anhydrous methanol into the 50L reaction kettle, stir To dissolve completely, control the temperature at 10-30°C;
[0032] (2) Decolorization: Add 11g of activated carbon into the reaction solution, stir and absorb for 30 minutes, filter the titanium rod for decarbonization, and pass the filtrate through a 0.22 μm microporous membrane for further decarbonization and sterilization, and keep the temperature of the filtrate at 10-30°C;
[0033] (3) Crystallization: the filtrate is transferred to the 50L crystallization tank in the 10,000-class local 100-class clean area, and slowly drips 5.48L of ethanol at a rate of 20L / H to the filtered filtrate under stirring conditions until the system is turbid. After cryst...
Embodiment 2
[0036] A kind of preparation method of novel anti-infective drug cefazedone sodium, the steps are as follows:
[0037] (1) Salt formation: Aseptically treat the reaction kettle, filter and utensils used, and add 1096.9g (2mol) cefazedone, 196.8g (2.4mol) sodium acetate and 6.58L anhydrous methanol into a 50L reaction kettle , stir to dissolve completely, and control the temperature at 10-30°C;
[0038] (2) Decolorization: Add 22g of activated carbon into the reaction solution, stir and absorb for 30 minutes, filter the titanium rod for decarbonization, and pass the filtrate through a 0.22μm microporous membrane for further decarbonization and sterilization, and keep the temperature of the filtrate at 10-30°C;
[0039] (3) Crystallization: the filtrate is transferred to the 50L crystallization tank in the 10,000-class local 100-class clean area, and slowly add 6.58L of ethanol dropwise to the filtered filtrate at a rate of 20L / H under agitation until the system is turbid. Afte...
Embodiment 3
[0042] A kind of preparation method of novel anti-infective drug cefazedone sodium, the steps are as follows:
[0043] (1) Salt formation: Aseptically treat the reaction kettle, filter and utensils used, and add 1096.9g (2mol) cefazedone, 213.2g (2.6mol) sodium acetate and 8.78L anhydrous methanol into the 50L reaction kettle , stir to dissolve completely, and control the temperature at 10-30°C;
[0044] (2) Decolorization: Add 32.9g of activated carbon into the reaction solution, stir and adsorb for 30 minutes, filter the titanium rod for decarbonization, and pass the filtrate through a 0.22μm microporous membrane for further decarbonization and sterilization, and keep the temperature of the filtrate at 10-30°C;
[0045] (3) Crystallization: the filtrate is transferred to the 50L crystallization tank in the local 100-class clean area of 10,000 grades, and slowly drips 8.77L of ethanol at a rate of 20L / H to the filtered filtrate under stirring conditions until the system bec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com