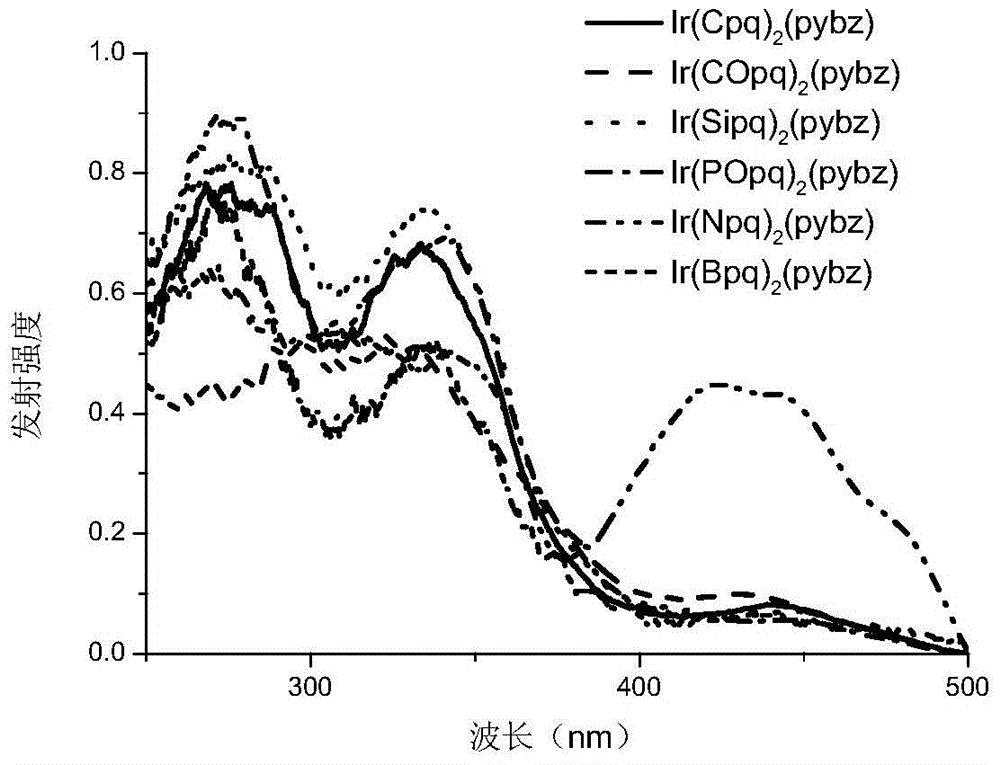
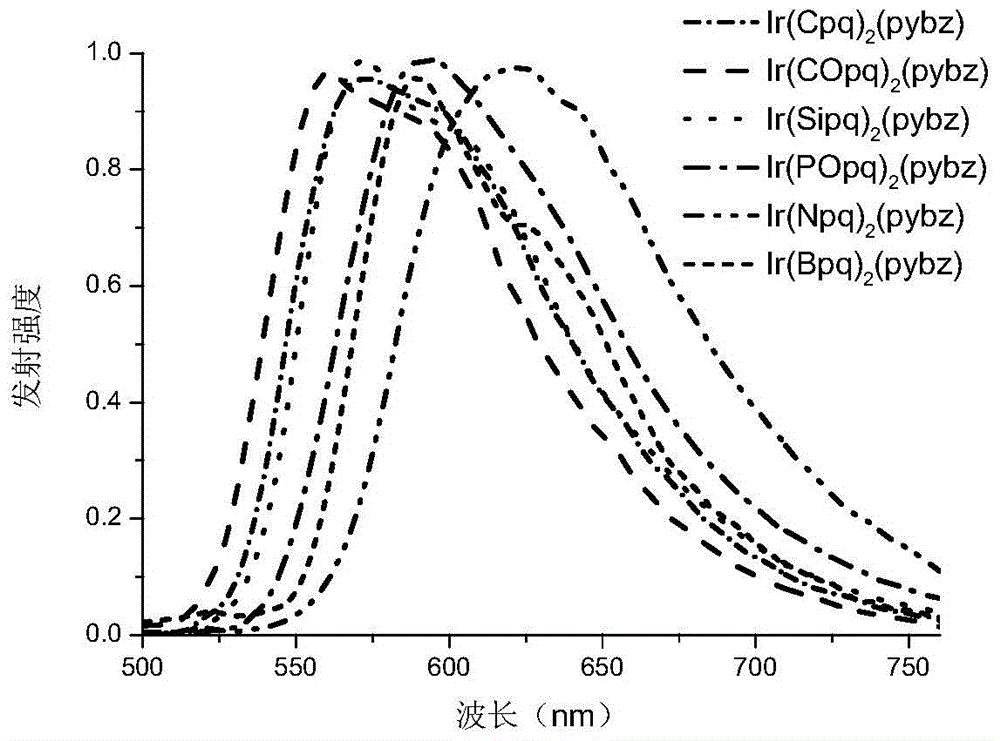
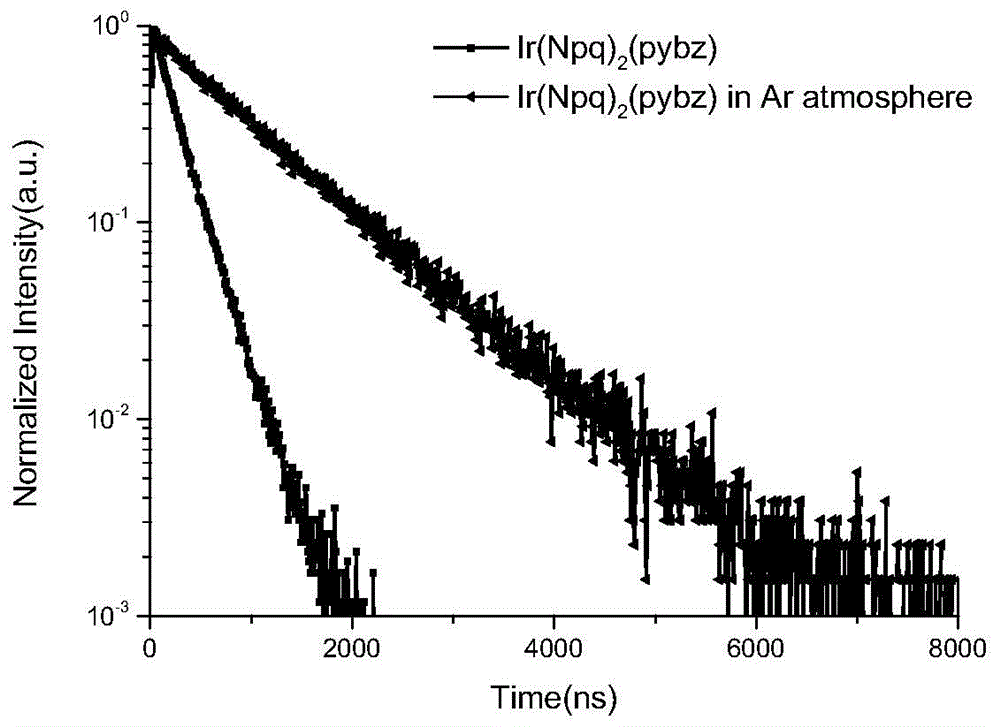
A kind of neutral iridium complex with bidentate ligand and its preparation method and application
A technology of iridium complexes and bidentate ligands, applied in the field of photoelectric functional organic materials, to avoid concentration quenching, simple synthesis steps, and improve carrier transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Complex Ir(Cpq) 2 Preparation of (pybz)
[0035] The reaction formula is as follows:
[0036]
[0037] 2-(4-Methylphenyl)quinoline (Cpq): Add 1.9g (12.5mmol) of o-nitrobenzaldehyde and 42.5mL of absolute ethanol into a round bottom flask, stir to dissolve it, then add 5g of iron powder ( 89.25mmol), 42.5mL of acetic acid and 21mL of distilled water, finally add a drop of concentrated hydrochloric acid, reflux for 15min (the reaction is complete as monitored by TLC) to stop the reaction, filter with suction, wash and filter with 100mL of water, collect the filtrate with CH 2 Cl 2 Extraction, the combined organic layers were separated with NaHCO 3 Aqueous and distilled water washes, anhydrous MgSO 4 Dry and concentrate to obtain anthranilaldehyde as a yellow oil. Add 5 mmol of anthranilaldehyde and 5 mmol of p-methylacetophenone to 20 mL of absolute ethanol, and then add saturated NaOH ethanol solution. The reaction mixture was heated and refluxed overnight, then ...
Embodiment 2
[0040] Complex Ir(COpq) 2 Preparation of (pybz)
[0041] The reaction formula is as follows:
[0042]
[0043] 2-(4-Methoxyphenyl)quinoline (COpq): Add 1.9g (12.5mmol) of o-nitrobenzaldehyde and 42.5mL of absolute ethanol in a round bottom flask, stir to dissolve it, and then add 5g of iron powder (89.25mmol), 42.5mL of acetic acid and 21mL of distilled water, finally add a drop of concentrated hydrochloric acid, reflux for 15min (the reaction is complete as monitored by TLC) to stop the reaction, filter with suction, wash and filter with 100mL of water, collect the filtrate with CH 2 Cl 2 Extraction, the combined organic layers were separated with NaHCO 3 Aqueous and distilled water washes, anhydrous MgSO 4 Dry and concentrate to obtain anthranilaldehyde as a yellow oil. Add 5 mmol of anthranilaldehyde and 5 mmol of p-methoxyacetophenone to 20 mL of absolute ethanol, and then add saturated NaOH ethanol solution. The reaction mixture was heated and refluxed overnight, ...
Embodiment 3
[0046] Compound Ir(Sipq) 2 Preparation of (pybz)
[0047] The reaction formula is as follows:
[0048]
[0049] 2-(4-triphenylsilylphenyl)quinoline (Sipq): Add 7.6g (50mmol) of o-nitrobenzaldehyde and 170mL of absolute ethanol into a round bottom flask, stir to dissolve it, and then add 20g of iron powder ( 357mmol), 170mL of acetic acid and 85mL of distilled water, finally add a drop of concentrated hydrochloric acid, reflux for 15min (the reaction is complete as monitored by TLC) to stop the reaction, filter with suction, wash and filter with 100mL of water, collect the filtrate with CH 2 Cl 2 Extraction, the combined organic layers were separated with NaHCO 3 Aqueous and distilled water washes, anhydrous MgSO 4 Dry and concentrate to give a yellow oil. Add 7.8mmol anthranilaldehyde and 7.8mmol p-bromoacetophenone to 30mL absolute ethanol, and then add saturated NaOH ethanol solution. The reaction mixture was heated and refluxed overnight, then cooled and filtered, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com