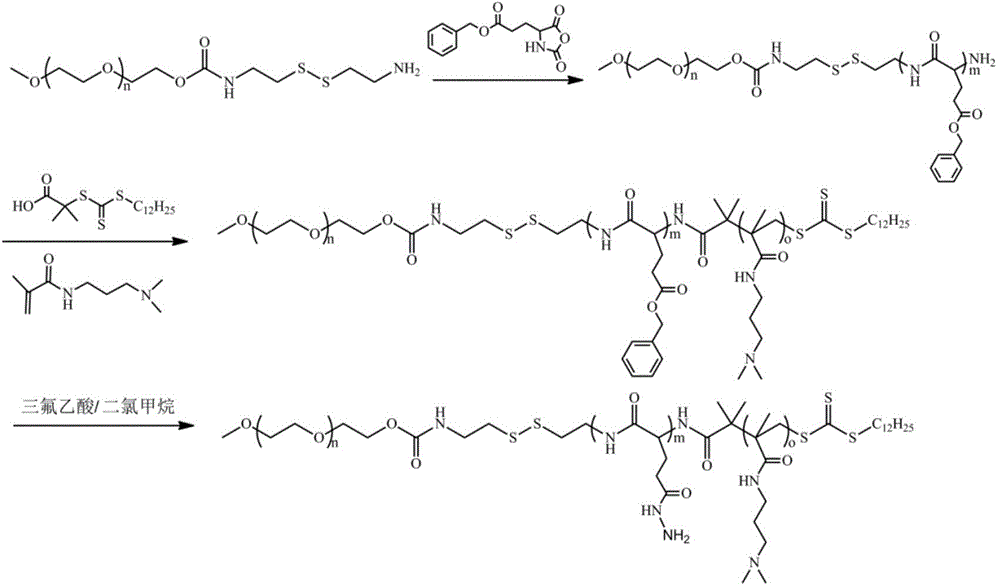
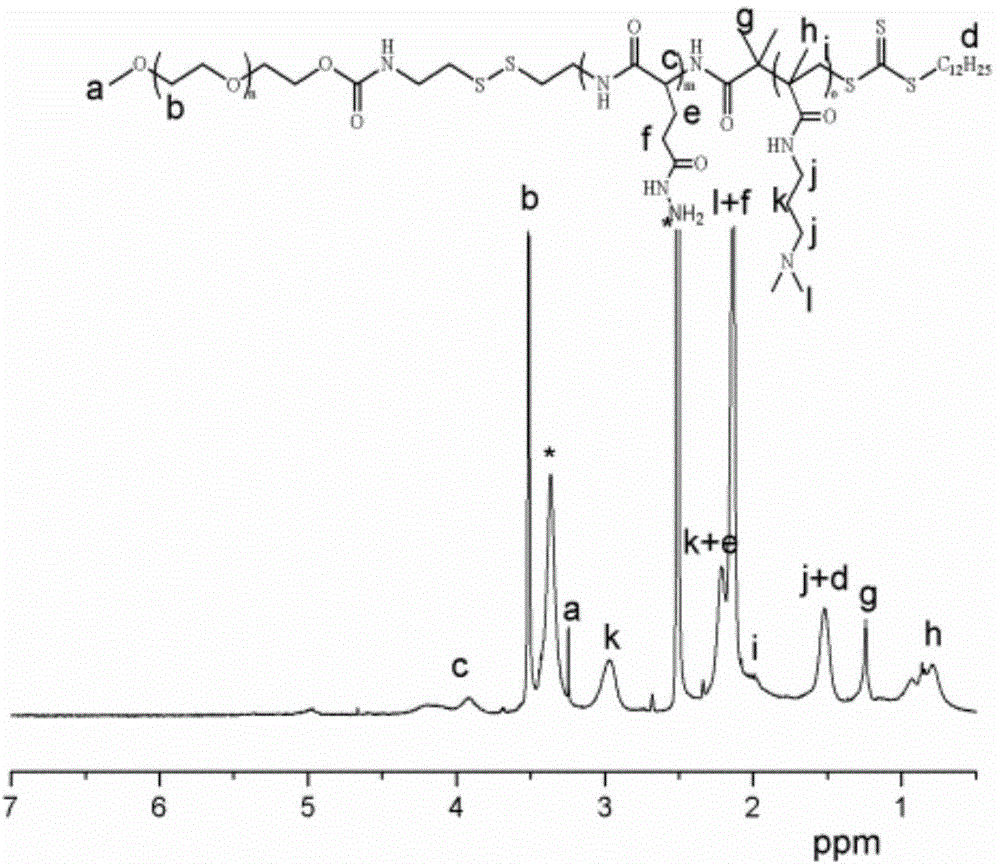
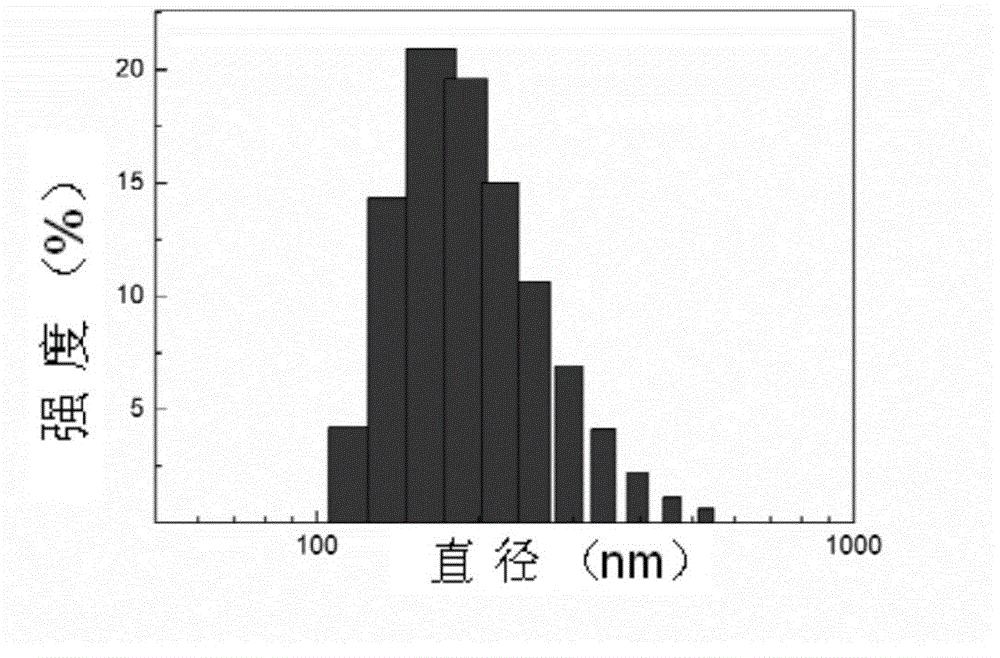
MPEG-poly(L-glutamic acid-gamma-hydrazide)-PDMAPMA triblock copolymer, and synthesis method and application thereof
A technology of block copolymers and synthesis methods, which is applied in the field of biomedical materials, can solve problems such as constraints, the impossibility of a single drug to achieve therapeutic effects, stability, and complicated schemes, so as to improve drug effects and avoid enzymatic hydrolysis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1) Synthesis of γ-benzyl ester-L-glutamic acid-N-carboxyl anhydride (BLG-NCA):
[0045] Add L-glutamic acid-γ-benzyl ester into anhydrous tetrahydrofuran, then add triphosgene under nitrogen atmosphere, then react at 50°C until a clear solution is formed, remove most of the solvent under reduced pressure to concentrate the solvent, and finally concentrate to obtain The reaction system was precipitated and recrystallized in anhydrous n-hexane to obtain BLG-NCA; wherein, the molar ratio of L-glutamic acid-γ-benzyl ester to triphosgene was 1:0.367, and every 100mL of anhydrous tetrahydrofuran Add 10 g of L-glutamic acid-γ-benzyl ester;
[0046] 2) Synthesis of cystaminated methoxypolyethylene glycol:
[0047] 2.1) Dissolve dry methoxy-terminated polyethylene glycol with a molecular weight of 2000, dibutyltin dilaurate and 2,2'-dithiodiethylisocyanate in anhydrous toluene, and react under a nitrogen atmosphere at 85°C For 48 hours, precipitate 3 times in anhydrous n-hexan...
Embodiment 2
[0064] 1) Synthesis of γ-benzyl ester-L-glutamic acid-N-carboxyl anhydride (BLG-NCA):
[0065] Add L-glutamic acid-γ-benzyl ester into anhydrous tetrahydrofuran, then add triphosgene under nitrogen atmosphere and react at 50°C to form a clear solution, remove most of the solvent under reduced pressure to concentrate the solvent, and finally concentrate the obtained The reaction system was precipitated and recrystallized in anhydrous n-hexane to obtain BLG-NCA; wherein, the molar ratio of L-glutamic acid-γ-benzyl ester to triphosgene was 1:0.4, and every 100mL of anhydrous tetrahydrofuran was added 15 g of L-glutamic acid-γ-benzyl ester;
[0066] 2) Synthesis of cystaminated methoxypolyethylene glycol:
[0067] 2.1) Dissolve dry methoxy-terminated polyethylene glycol with a molecular weight of 4000, dibutyltin dilaurate and 2,2'-dithiodiethylisocyanate in anhydrous toluene, and react under a nitrogen atmosphere at 85°C For 48 hours, precipitate 3 times in anhydrous n-hexane, ...
Embodiment 3
[0078] 1) Synthesis of γ-benzyl ester-L-glutamic acid-N-carboxyl anhydride (BLG-NCA):
[0079] Add L-glutamic acid-γ-benzyl ester into anhydrous tetrahydrofuran, then add triphosgene under nitrogen atmosphere, then react at 50°C until a clear solution is formed, remove most of the solvent under reduced pressure to concentrate the solvent, and finally concentrate to obtain The reaction system was precipitated and recrystallized in anhydrous n-hexane to obtain BLG-NCA; wherein, the molar ratio of L-glutamic acid-γ-benzyl ester to triphosgene was 1:0.383, and every 100mL of anhydrous tetrahydrofuran Add 12g of L-glutamic acid-γ-benzyl ester;
[0080] 2) Synthesis of cystaminated methoxypolyethylene glycol:
[0081] 2.1) Dissolve dry methoxy-terminated polyethylene glycol with a molecular weight of 1000, dibutyltin dilaurate and 2,2'-dithiodiethylisocyanate in anhydrous toluene, and store at 85°C under a nitrogen atmosphere. React for 48 hours, precipitate 3 times in anhydrous n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com