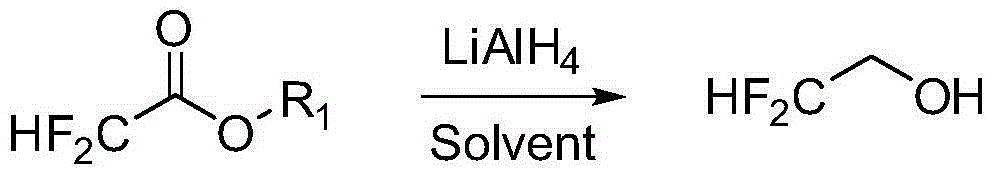
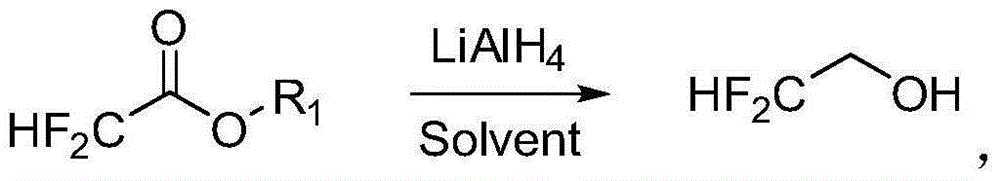
Preparation method of 2, 2-difluoroethanol
A technology of difluoroethanol and ethyl difluoroacetate is applied in the field of preparation of 2,2-difluoroethanol, and can solve the problems of low yield, decrease in total yield, increase production cost and the like, and achieve reduction in production cost, Emission reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] ·In a 2000mL three-necked flask, dissolve 38.0g (1.0mol) LiAlH4 with 800ml tetrahydrofuran. Cool the system to -10°C in an ice bath, stir vigorously, and slowly drop 248 g (2.0 mol) of ethyl difluoroacetate into the three-necked flask. After the dropwise addition, react for 1 hour. The NMR spectrum confirms that the conversion is complete. Use 2 mol / L hydrochloric acid to adjust the pH to about 3 to terminate the reaction. Difluoroethanol can be obtained by distillation.
[0027] • In a 2000 mL three-necked flask, dissolve 38.0 g (1.0 mol) LiAlH4 with 800 ml ethylene glycol dimethyl ether. Cool the system to -10°C in an ice bath, stir vigorously, and slowly drop 248 g (2.0 mol) of ethyl difluoroacetate into the three-necked flask. After the dropwise addition, react for 1 hour. The NMR spectrum confirms that the conversion is complete. Use 2 mol / L hydrochloric acid to adjust the pH to about 3 to terminate the reaction. Difluoroethanol can be obtained by distillation.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com