Purity determination method for gonadotropin
A gonadotropin and determination method technology, applied in measurement devices, instruments, scientific instruments, etc., can solve problems such as inability to separate effectively, the molecular weight difference is not very large, and the main peak and impurity peak cannot be effectively separated.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0130] Preparation of Gonadotropin APIs
[0131] The inventor has also obtained a method for preparing gonadotropin bulk drugs, said method comprising the following steps:
[0132] A, with the method for measuring the purity as described in the first aspect of the present invention, measure the total impurity content in the former powder of gonadotropins;
[0133] B. If the measurement result of step (A) shows that the total impurity content in the raw powder is > 2%, then the raw powder is repurified until the measurement result shows that the total impurity content is ≤ 2%;
[0134] C. If the measurement result of step (A) shows that the total impurity content in the raw powder is ≤ 2%, then add a pharmaceutically acceptable amount of excipients to the raw powder of gonadotropins to prepare raw materials of gonadotropins medicine.
[0135] By using the determination method provided by the inventor, the purity of the gonadotropin raw powder is analyzed and determined, so as...
Embodiment 1
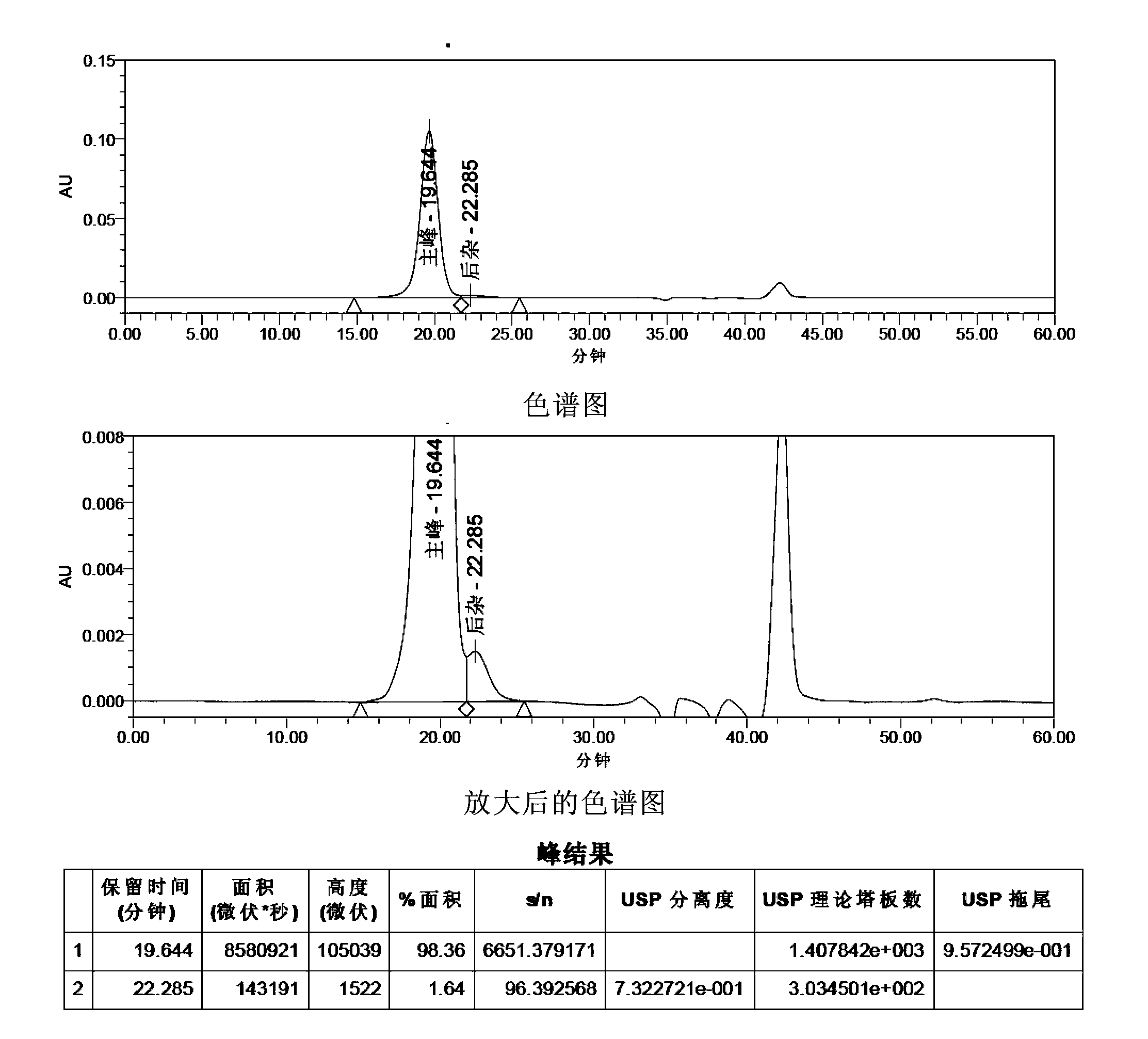
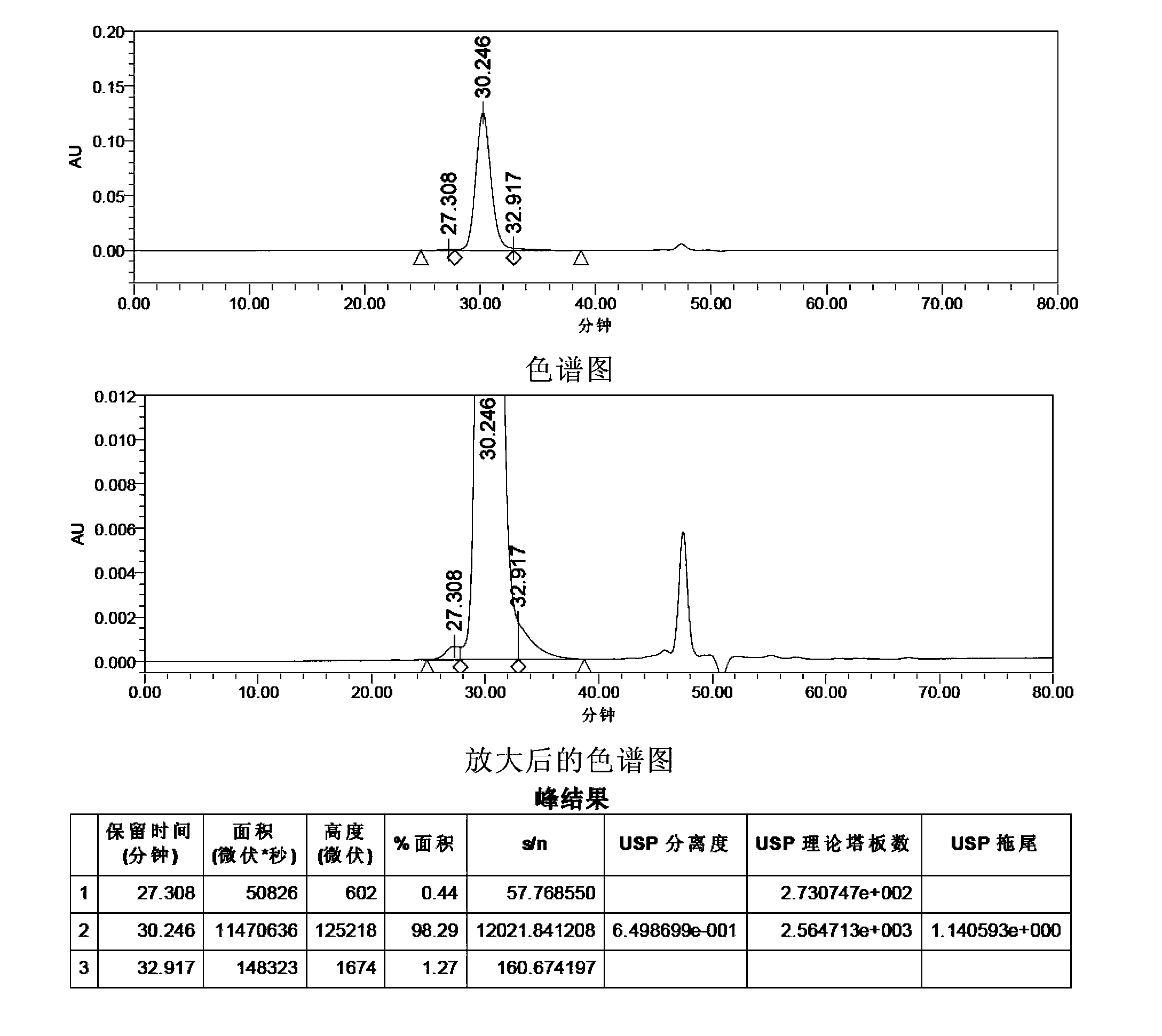
[0152] HPLC Purity Analysis of FSH API
[0153] Sample to be analyzed: FSH API (produced by Shanghai Tianwei Bio-Pharmaceutical Co., Ltd., batch number: 111201, biological potency in terms of FSH 256.8IU / mg)
[0154] Method 1: This method adopts the liquid chromatography method of the literature "Compositional Analyzes of a Human Menopausal Gonadotrophin Preparation Extracted from Urine (menotropin)" to carry out the HPLC purity analysis of FSH.
[0155] Mobile phase: Take 71.6g of disodium hydrogen phosphate, add 700ml of water to dissolve, adjust the pH value to 7.0 with phosphoric acid, add water to 800ml, add 200ml of acetonitrile, mix, filter, and ultrasonically degas.
[0156] Get FSH crude drug 28.90mg, dissolve with mobile phase and make the solution that contains 500 units in every 1ml calculated by the marked amount, as need testing solution. Measured according to high performance liquid chromatography (Appendix VD of Part Two of the Chinese Pharmacopoeia 2010 Editi...
Embodiment 2
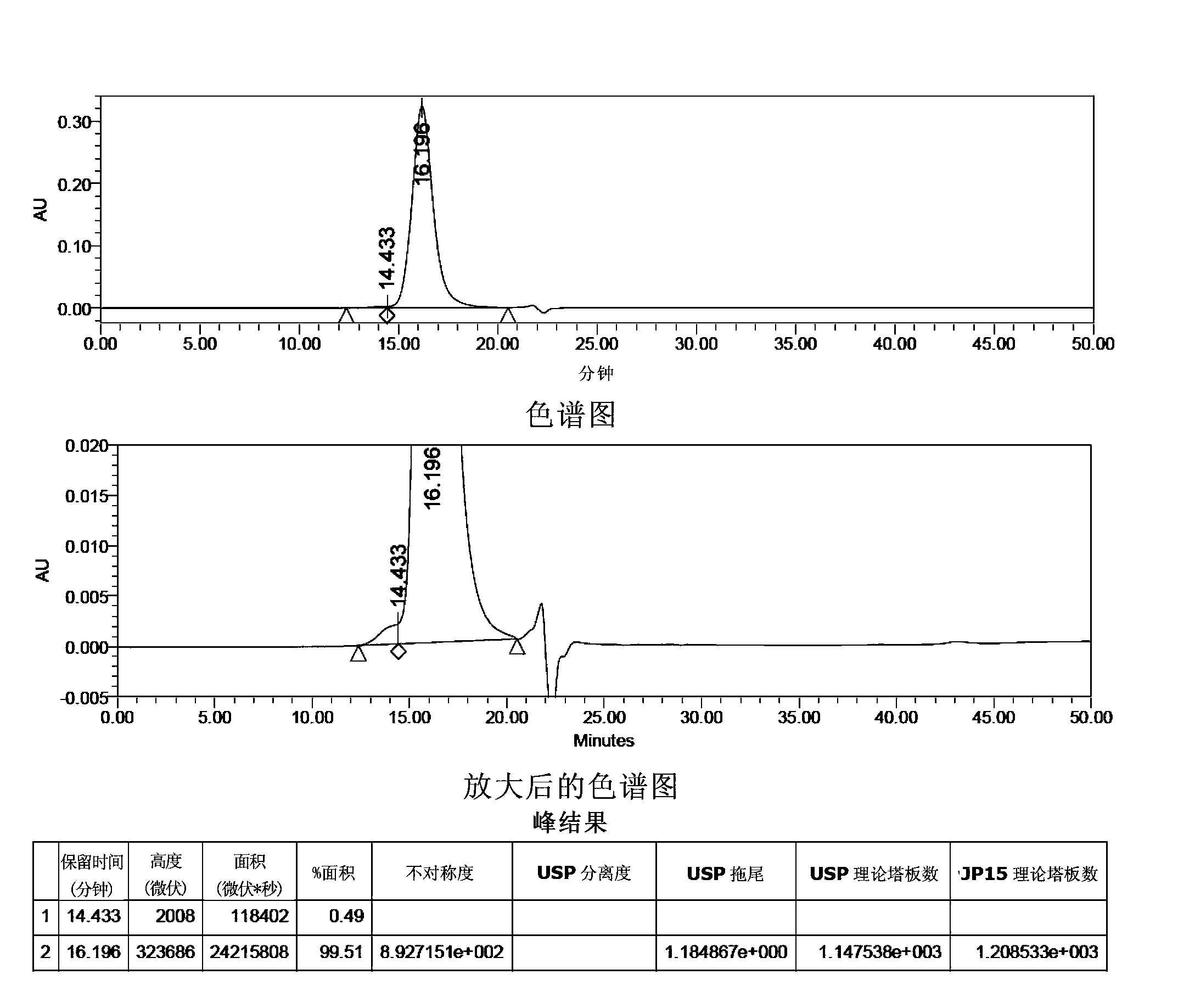
[0171] HPLC Purity Analysis of FSH API
[0172] Sample to be analyzed: FSH raw material drug (provided by Shanghai Tianwei Biopharmaceutical Co., Ltd., batch number: 100401, biological potency is 289.5IU / mg in terms of FSH)
[0173]Method 1: In this method, a TSK G2000SWXL (7.8×300mm) chromatographic column and a Superdex75 10 / 300GL chromatographic column are used for liquid chromatography in series to analyze the HPLC purity of FSH.
[0174] Mobile phase: 50mM sodium dihydrogen phosphate, pH7.0-acetonitrile (80:20).
[0175] Get FSH crude drug 28.72mg, dissolve with mobile phase and make the solution that contains 3500 units in every 1ml calculated by labeling amount, as need testing solution. Determined with reference to high-performance liquid chromatography (Appendix VD of Part Two of the Chinese Pharmacopoeia 2010 Edition). TSK G2000SWXL (7.8×300mm) and Superdex75 10 / 300GL are connected in series as the analytical chromatographic column, the flow rate is 0.5ml per minut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com