Synthesis of Lorcaserin and preparation method of intermediate of Lorcaserin
A technology of intermediates and preparation steps, which is applied in the field of pharmaceuticals and can solve problems such as irritation and corrosion, environmental pollution, and no advantages in industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、1
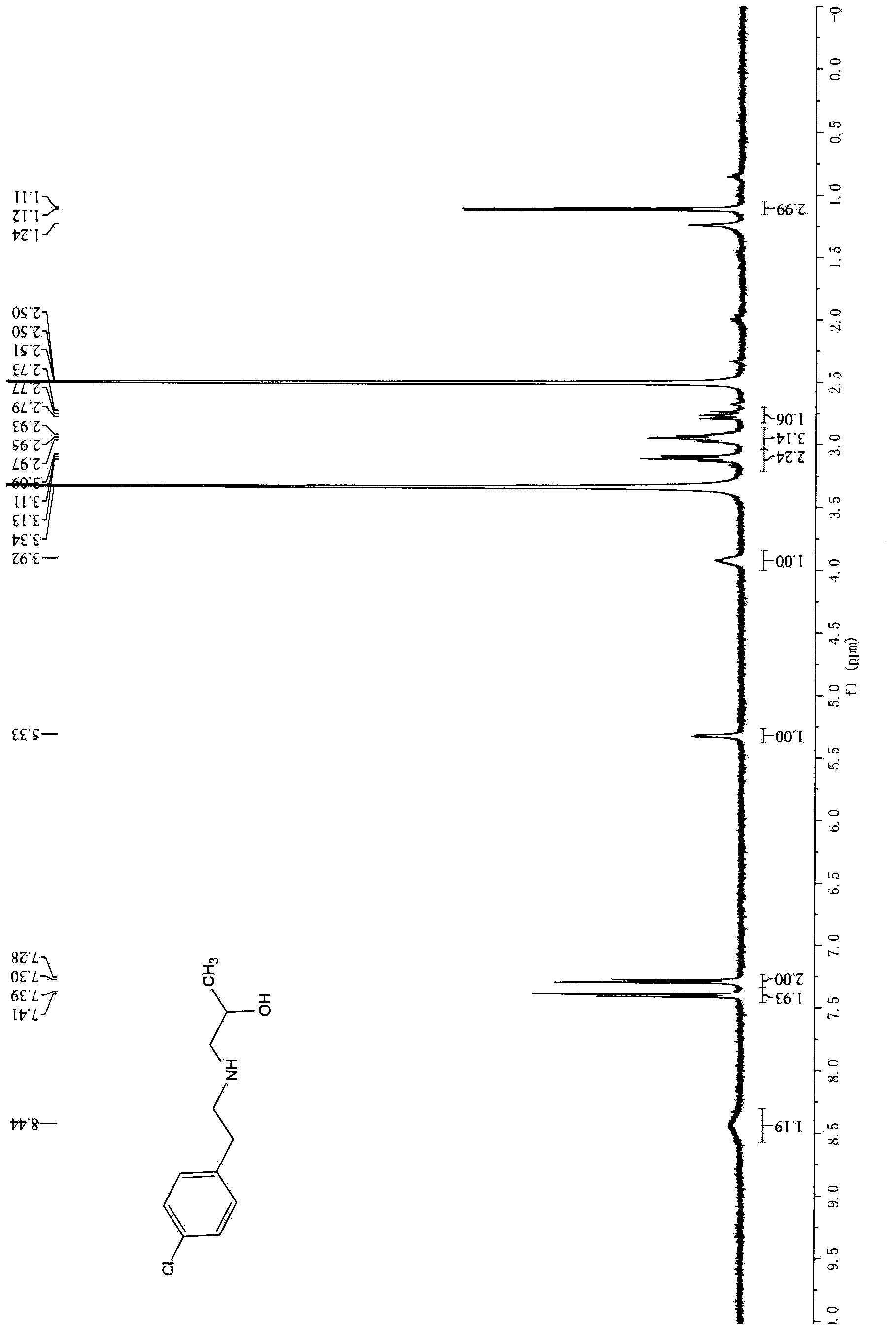
[0021] Embodiment 1, the preparation of 1-[[2-(4-chlorophenyl) ethyl] amino]-2-hydroxypropane (formula I compound)
[0022] Add 30kg of water into a 50L reactor, stir, add 373.2g of propylene oxide at room temperature, stir evenly, then slowly add 1000g of p-chlorophenethylamine, stir slowly to 90±5°C, and react for 5 hours. Cool down to room temperature, add dichloromethane 15L×3 for extraction three times, combine the dichloromethane layers, dry with 1kg anhydrous sodium sulfate overnight, filter, and concentrate the filtrate to dryness to obtain 1250g of light yellow solid. Yield 91%.
[0023] 1 HNMR (d6-DMSO, 400Hz) δ (ppm): 8.44 (br, 1H), 7.39 ~ 7.41 (d, 2H), 7.28 ~ 7.30 (d, 2H), 5.33 (d, 1H), 3.92 (m, 1H ) 3.09-3.13 (t, 2H), 2.93-2.97 (m, 3H), 2.73-2.79 (m, 1H), 1.11-1.12 (d, 3H).
Embodiment 2、1
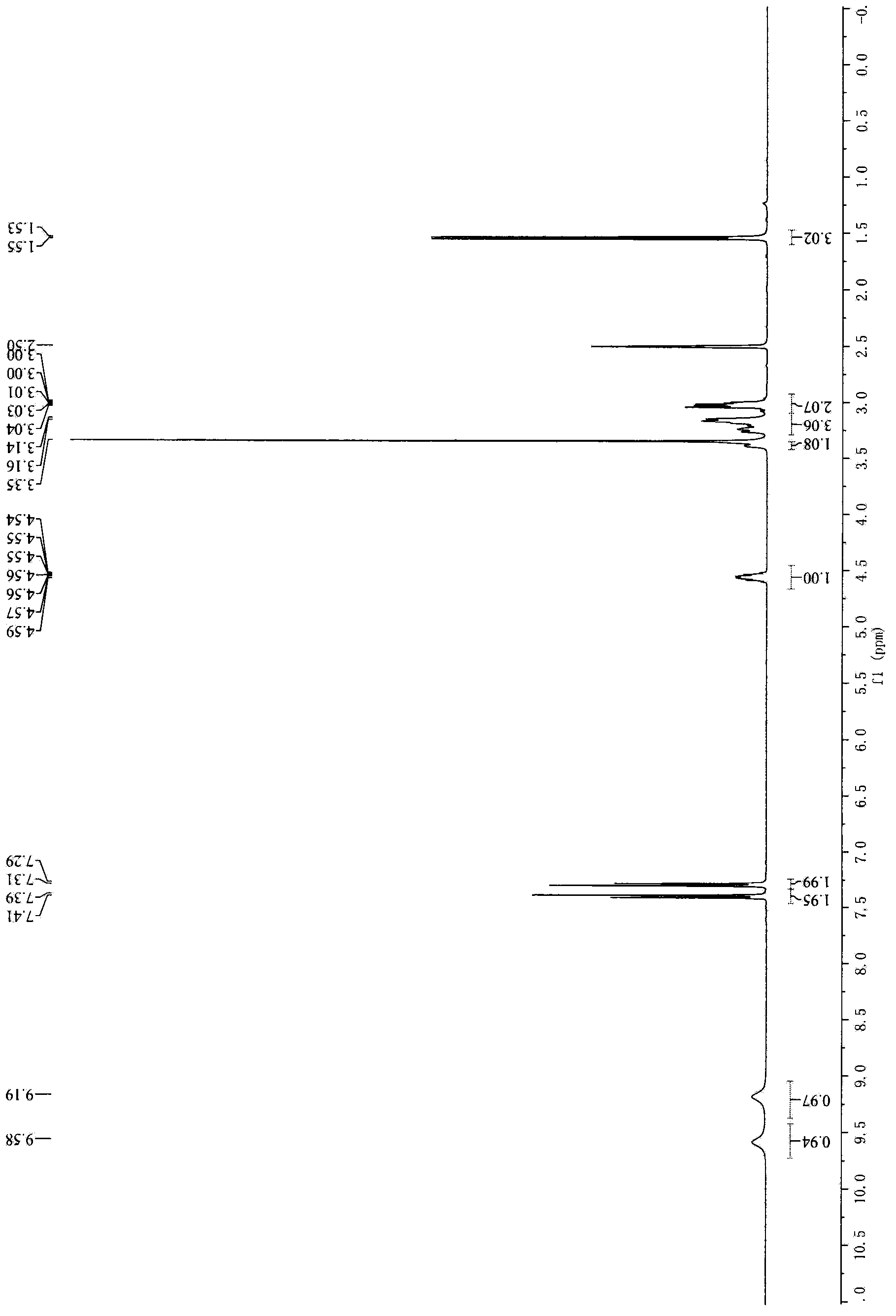
[0024] Embodiment 2, the preparation of 1-[[2-(4-chlorophenyl) ethyl] amino]-2-chloropropane hydrochloride
[0025] Add 500ml of toluene, 75g of 1-[[2-(4-chlorophenyl)ethyl]amino]-2-hydroxypropane, 40g of DMF, and 250g of thionyl chloride into a 2L three-necked flask, and mix well until the reaction is at 65°C , Dissolving clear (colorless) at 40 ° C, with the increase of temperature, the color gradually deepens. After reacting for three hours, TLC (DCM:MeOH=10:1) detected that the starting material was completely reacted. Stop heating, cool down in an ice-water bath to below 10°C, filter with suction, wash with 300ml*2 toluene, beat the filter cake with 1000ml isopropanol overnight. The next day, it was filtered with suction, washed with 200ml×2 isopropanol, and dried in an oven at 40°C for 4h to obtain 63g of white solid. Yield 73.8%.
[0026] 1 HNMR (d6-DMSO, 400Hz) δ (ppm): 9.58 (br, 1H), 9.19 (br, 1H), 7.39 (d, 2H), 7.31 (d, 2H), 4.54~4.59 (m, 1H), 3.39 (m, 1H), 3.24...
Embodiment 3、8
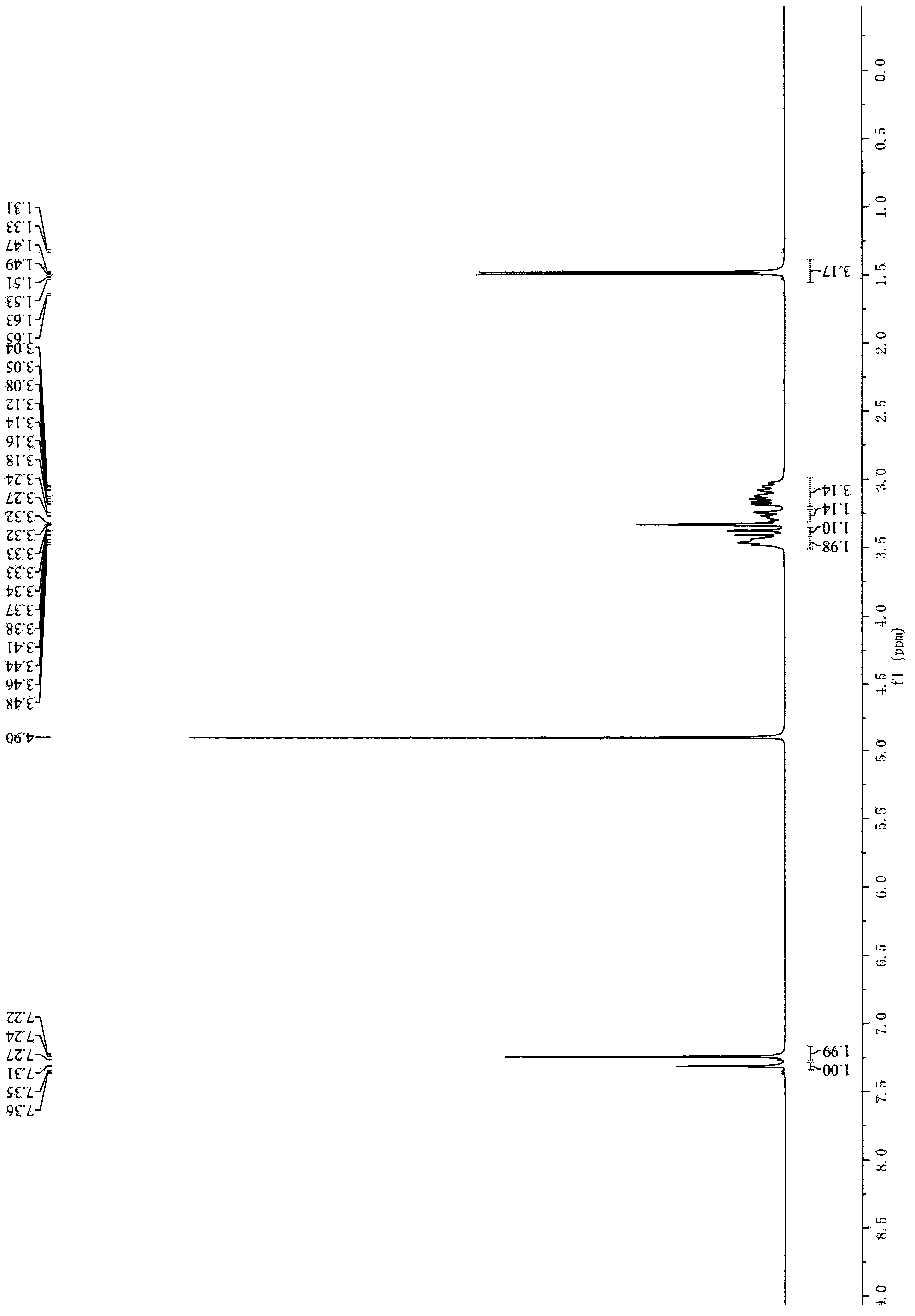
[0027] Example 3, Preparation of 8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine
[0028] Add 600ml of 1,2-dichlorobenzene to a 2000ml reaction flask, then add 60g of 1-[[2-(4-chlorophenyl)ethyl]amino]-2-chloropropane hydrochloride successively, and trichloride 80g of aluminum is heated up to 130°C for reaction. The raw material dissolves at 60°C, and dissolves at 110°C. TLC (DCM:MeOH=10:1) detected the disappearance of the starting material, stopped the heating, and naturally cooled to room temperature. Slowly add 800ml of 1N HCl solution and 400ml of methyl tert-butyl ether, stir, separate the layers, and extract the organic phase with 400mL of 1N HCl solution×2. Combine the aqueous phases, adjust the pH>11 with 30% sodium hydroxide solution, extract with 800ml×3 methyl tert-butyl ether, combine the organic phases, dry over anhydrous sodium sulfate, filter, and spin dry. 64 g of crude product were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com