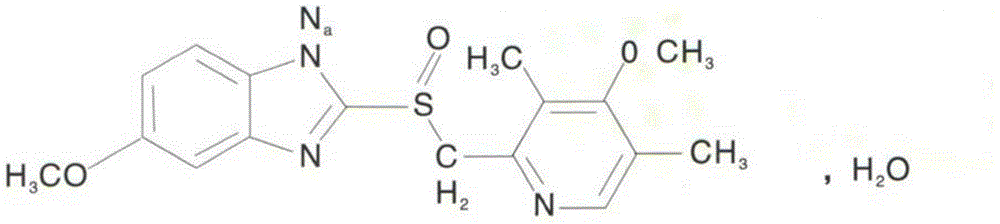
Special ultrafine omeprazole sodium powder freeze-dried preparation and preparation method thereof
A technology of omeprazole sodium and ultrafine powder, applied in the field of medicine, can solve the problems of large toxic and side effects, small specific surface area, low clarity, etc., and achieve the effect of improving utilization rate, large specific surface area and good clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 222 grams (1mol) of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride and 180 grams (1mol) of 2-mercapto-5-methoxy-1H-benzimidazole Add to 5 liters of ethanol and acetone mixed solvent, the volume ratio of ethanol and acetone is 3:1, the mixed solvent contains 80 grams (2mol) of sodium hydroxide, while adding 4.5 grams (0.03mol) of sodium iodide catalyst, this reaction The mixture was heated to reflux for 1.5 hours, cooled to room temperature, filtered to remove insoluble matter, the filtrate was distilled off under reduced pressure to remove most of the solvent, the residue was dissolved by adding 3 liters of ethyl acetate, washed twice with 1 liter of water, washed with anhydrous sodium sulfate Dry the organic phase, filter, concentrate, add 400ml of acetone to the residue, freeze the organic phase to 0°C, precipitate a solid overnight, and filter to obtain 315.8 g of off-white solid 5-methoxy-2-[(4-methoxy-3 , 5-dimethylpyridin-2-yl)methylthio]-1H-benzimida...
Embodiment 2
[0035] 222 grams (1mol) of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride and 180 grams (1mol) of 2-mercapto-5-methoxy-1H-benzimidazole Add to 5 liters of ethanol and acetone mixed solvent, the volume ratio of ethanol and acetone is 3:1, the mixed solvent contains 80 grams (2mol) of sodium hydroxide, while adding 3.7 grams (0.025mol) of sodium iodide catalyst, this reaction The mixture was heated to reflux for 2 hours, cooled to room temperature, filtered to remove insoluble matter, the filtrate was distilled under reduced pressure to remove most of the solvent, the residue was dissolved in 3 liters of ethyl acetate, washed twice with 1 liter of water, washed with anhydrous sodium sulfate Dry the organic phase, filter, concentrate, add 400ml of acetone to the residue, freeze the organic phase to 0°C, precipitate a solid overnight, and filter to obtain 315.8 g of off-white solid 5-methoxy-2-[(4-methoxy-3 , 5-dimethylpyridin-2-yl)methylthio]-1H-benzimidazole.
[00...
Embodiment 3
[0041] 222 grams (1mol) of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride and 180 grams (1mol) of 2-mercapto-5-methoxy-1H-benzimidazole Add to 5 liters of ethanol and acetone mixed solvent, the volume ratio of ethanol and acetone is 3:1, the mixed solvent contains 80 grams (2mol) of sodium hydroxide, while adding 3 grams (0.02mol) of sodium iodide catalyst, this reaction The mixture was heated to reflux for 2.5 hours, cooled to room temperature, filtered to remove insoluble matter, the filtrate was distilled under reduced pressure to remove most of the solvent, and 3 liters of ethyl acetate was added to the residue to dissolve, washed twice with 1 liter of water, washed with anhydrous sodium sulfate Dry the organic phase, filter, concentrate, add 400ml of acetone to the residue, freeze the organic phase to 0°C, precipitate a solid overnight, and filter to obtain 315.8 g of off-white solid 5-methoxy-2-[(4-methoxy-3 , 5-dimethylpyridin-2-yl)methylthio]-1H-benzimidaz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com