Application of bionic reconstituted high-density lipoprotein in preparation of drugs for prevention and treatment of Alzheimer disease
A high-density lipoprotein and Alzheimer's disease technology, applied in the fields of neuropharmacology and chemical pharmacy, can solve problems such as the application of AD prevention and treatment, and achieve the effect of promoting extra-brain transport and reducing inflammatory response in the brain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Characterization of recombinant high-density lipoprotein
[0030] (1) Preparation
[0031] Lipid (phosphatidylcholine + / - ganglioside + / - cholesterol + / - cholesterol oleate) (2-10 mg) was dissolved in chloroform, and the organic solvent was removed by rotary evaporation under reduced pressure, and the lipid film was added to pH 7.4 Hydrate with phosphate buffer and homogenize with ultrasound at 50°C. Add 0.5-5 mg of ApoE3 and continue to sonicate for 50 min. The product was cooled to room temperature, incubated overnight, and stored at 4°C for later use.
[0032] (2) Characterization
[0033] Recombinant high-density lipoprotein was negatively stained with phosphotungstic acid, and its morphology was observed with a transmission electron microscope. Laser particle size analyzer was used to measure the particle size and surface potential. Analysis of recombinant high-density lipoprotein components: Fluorescence spectrophotometer to measure the amount of en...
Embodiment 2
[0035] Example 2 Recombinant high-density lipoprotein binding Aβ 1-40 monomer, oligomer
[0036] (1) Preparation
[0037] Weigh soy phospholipids, egg phospholipids (2-10 mg) and 0.02 mg fluorescent probe DiI into a round bottom flask, add chloroform to dissolve, place in a rotary evaporator at 20 ° C, and remove the organic solvent under vacuum for 1 h in the dark. Add PBS solution to a round bottom flask, shake at 37°C until the lipid film on the inner wall of the flask is completely hydrated and fall off, ultrasonically reduce the particle size at 40°C, add ApoE or ApoE mimic peptide (0.1-10mg), incubate at 37°C for 36h, 4 Store at ℃.
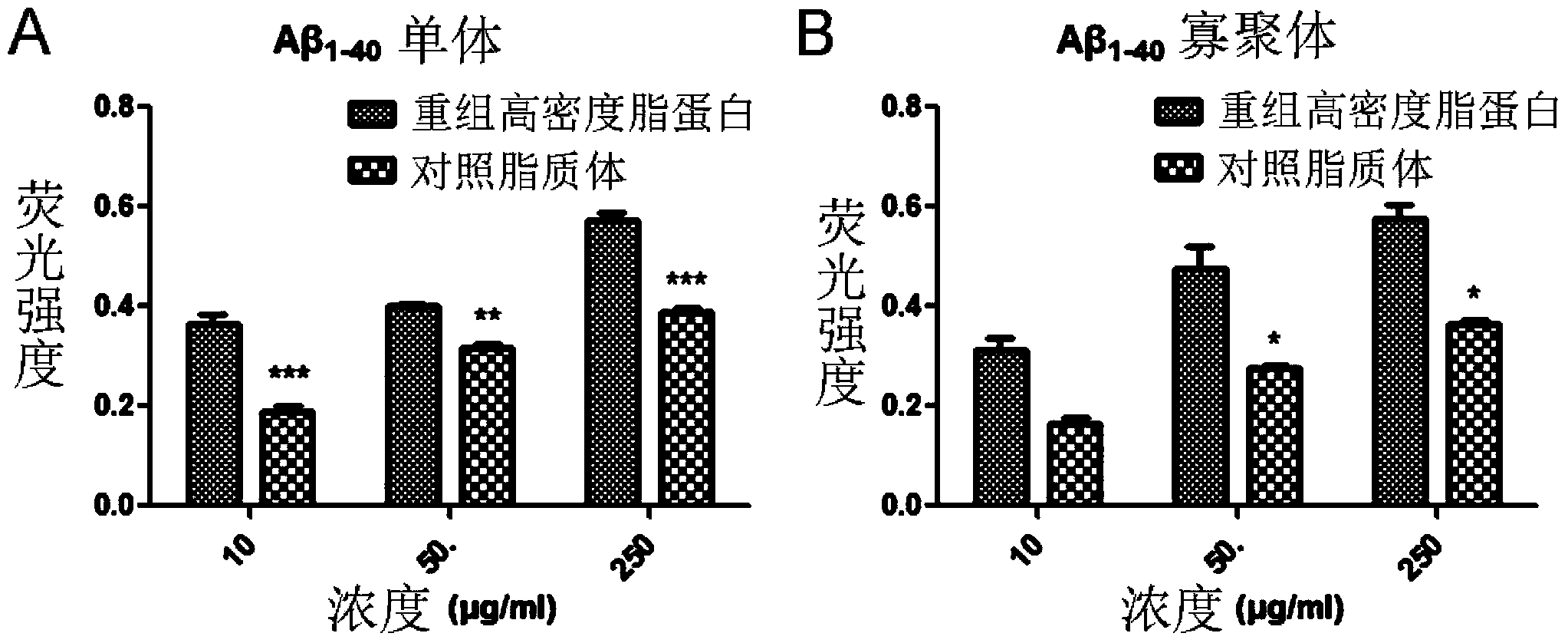
[0038] (2) Recombinant HDL and Aβ 1-40 In vitro adsorption and binding experiments of monomers and oligomers
[0039] Aβ 1-40 0.05 M pH9.6 sodium carbonate-sodium bicarbonate solution of monomer (concentration: 500 μg / ml) or oligomer (concentration: 500 μg / ml) was added to the plate wells, 50 μl per well, and incubated overnight at 4°C....
Embodiment 3
[0040] Example 3 Aβ-affinity properties of recombinant high-density lipoprotein
[0041] (1) Preparation
[0042] Weigh phosphatidylcholine and phosphatidic acid (2-10 mg) into a round bottom flask, add chloroform to dissolve, and place in a rotary evaporator to remove the organic solvent under reduced pressure. Add PBS solution to the round-bottomed flask, shake at 37°C until the lipid film on the inner wall of the flask is completely hydrated and fall off, homogeneously reduce the particle size at 40°C, add ApoE or ApoA-I (0.1-10mg), incubate at 37°C for 36h, Store at 4°C.
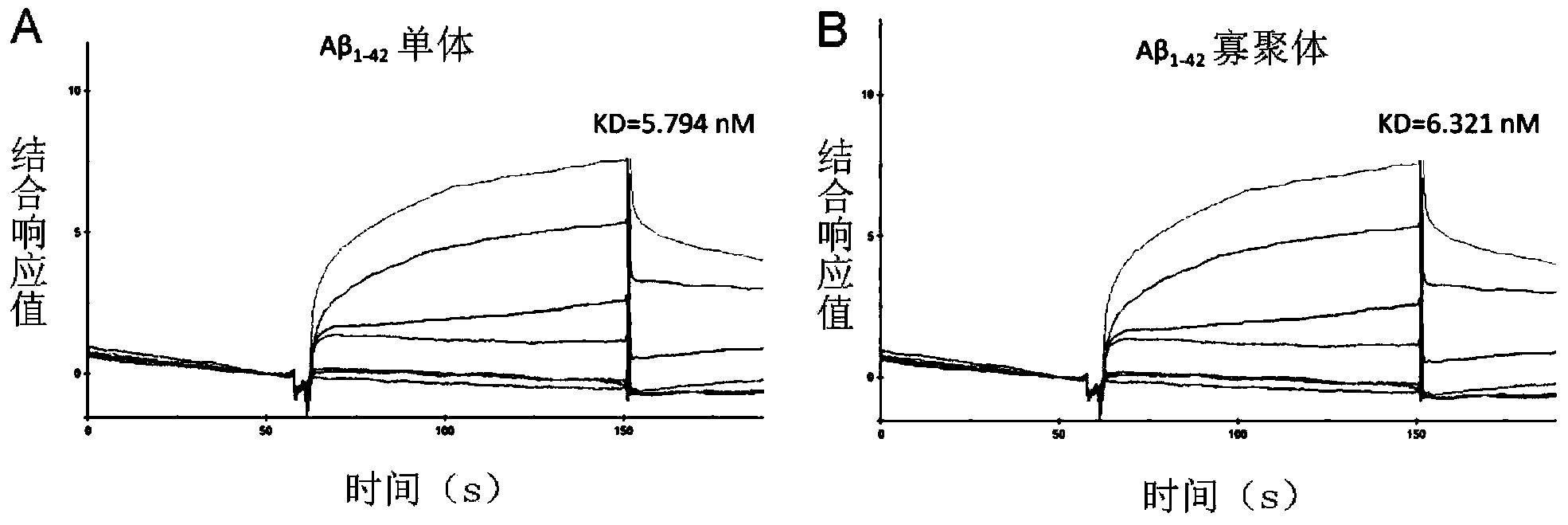
[0043] (2) The surface plasmon resonance (Surface Plasmon Resonance, SPR) experiment verified the Aβ affinity properties of the recombinant HDL.
[0044] The CM5 chip immobilizes Aβ monomers or oligomers by amino coupling: after activating the surface of the chip with 0.2MEDC and 0.05M NHS, dilute Aβ monomers or oligomers in pH4.0 sodium acetate buffer solution In this method, the concentration of Aβ ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com