A beta targeted recombinant lipoprotein nano drug carrier and preparation method and application thereof
A nano-drug carrier and lipoprotein technology, which is applied in the direction of drug combination, pharmaceutical formula, preparations for in vivo tests, etc., to achieve good safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
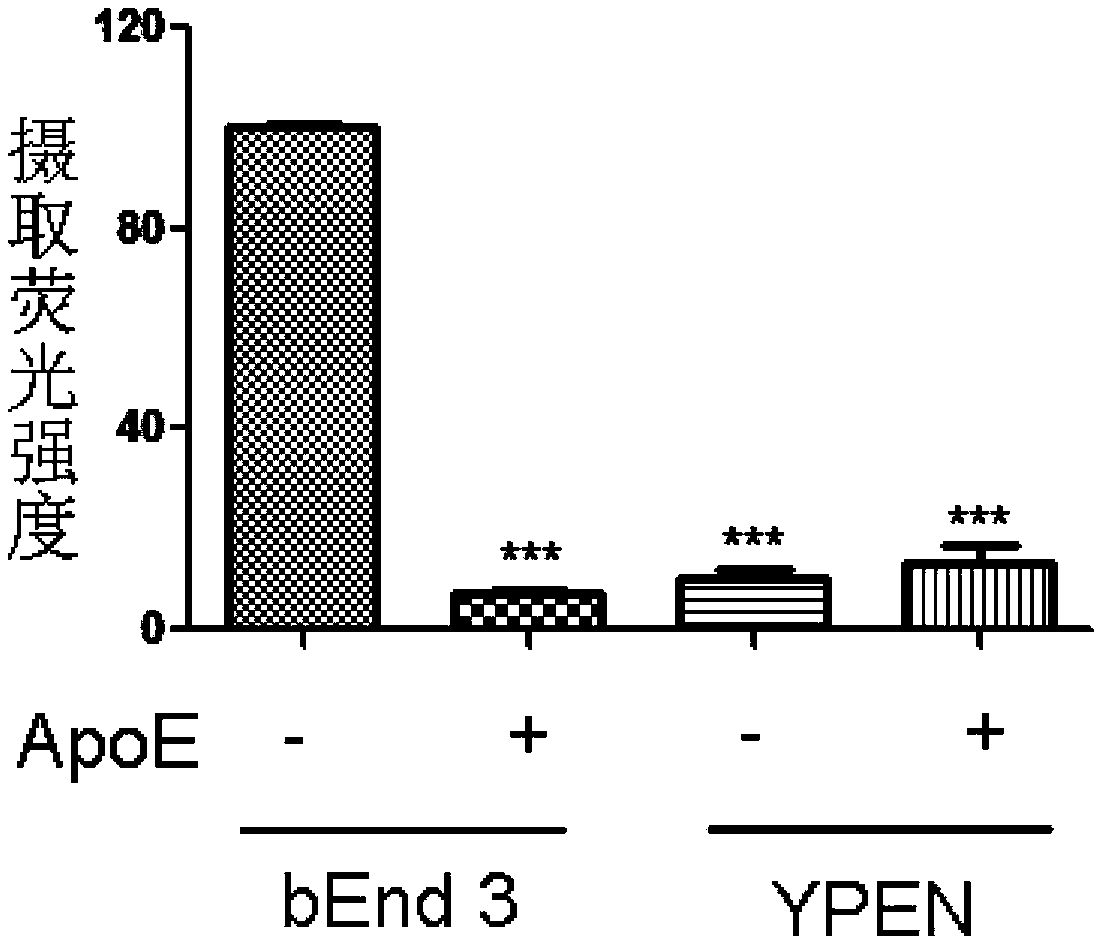
[0027] Example 1 Characterization of recombinant lipoproteins and uptake by cerebrovascular endothelial cells
[0028] (1) Preparation
[0029] Lipid (phosphatidylcholine DMPC / DOPC / DPPC+ / -ganglioside GM1+ / -cholesterol+ / -cholesterol oleate) (2-10mg), drug α-mangostin (0.1-2mg) dissolved in a certain Proportional chloroform-methanol mixed solvent, remove the organic solvent by rotary evaporation under reduced pressure, add 1ml Tris buffer (containing 10mM Tris, 0.1M KCl, 1mM EDTA, pH8.0 degassed) to the lipid film for hydration, 50°C water bath ultrasonic 1.5h . Add buffers of different pH and salt composition to dilute to 8ml, and sonicate in a water bath at 40°C. ApoE3 (0.5-5 mg) was stored in 100 mM ammonium bicarbonate solution (pH 8.0) containing 6M guanidine hydrochloride and 5 μl / (mg protein) β-mercaptoethanol. Dialyze against the appropriate protein assembly buffer before use. Add within 10 minutes, and continue to sonicate for 50 minutes. The product was cooled to ...
Embodiment 2
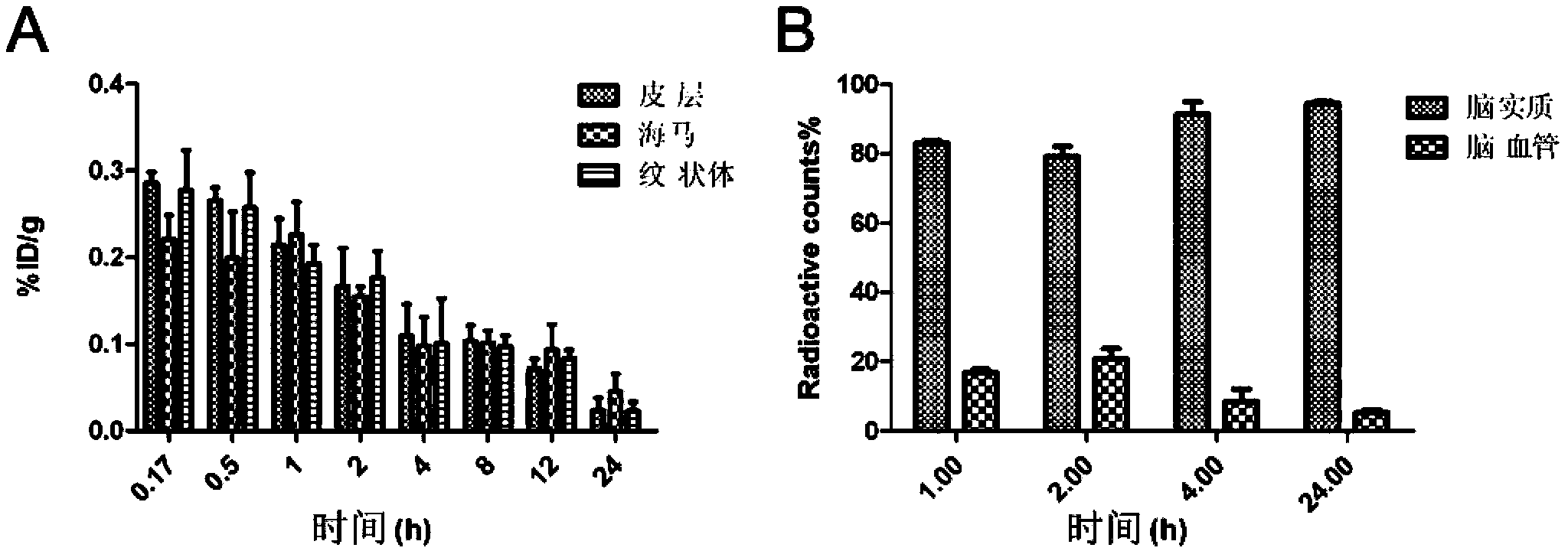
[0035] Example 2 Intracerebral Delivery Properties of Recombinant Lipoproteins
[0036] (1) Preparation
[0037] Weigh soybean lecithin, egg lecithin (2-10mg), ganglioside GM1 (0.1-5mg) into a 500ml round bottom flask, add 2ml of chloroform to dissolve, place in a rotary evaporator at 20°C, avoid light and vacuum for 1h. Add 2ml of PBS solution to the round bottom flask, shake at 37°C until the lipid film on the inner wall of the flask is completely hydrated and fall off, put it in a water bath at 40°C and sonicate for 1 hour, add ApoE or ApoA-I (0.1-10mg), incubate at 37°C for 36 hours, Store at 4°C.
[0038] (2) Quantitative determination of the distribution of recombinant lipoprotein in various tissues and different brain regions in the body after intravenous injection of radiolabeled
[0039] 125 I labeled recombinant lipoproteins. Twenty-four Kunming mice were randomly divided into 8 groups and administered intravenously 125 I-recombinant lipoprotein, the animals wer...
Embodiment 3
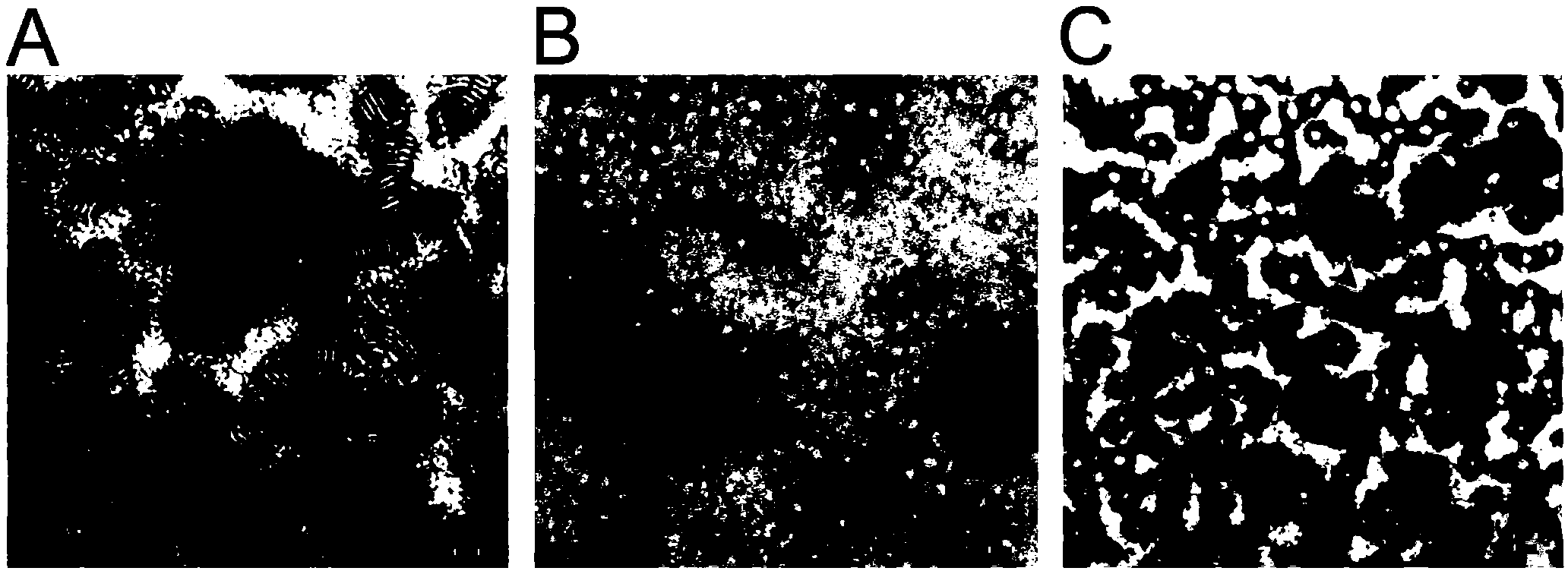
[0040] Example 3 Recombinant lipoprotein and Aβ 1-40 oligomer binding site
[0041] (1) Preparation
[0042] Weigh phosphatidylcholine and phosphatidic acid (2-10mg) into a 500ml round bottom flask, add 2ml of chloroform to dissolve, place in a rotary evaporator at 20°C, and vacuum for 1h in the dark. Add 2ml of PBS solution to the round bottom flask, shake at 37°C until the lipid film on the inner wall of the flask is completely hydrated and fall off, put it in a water bath at 40°C and sonicate for 1 hour, add ApoE or ApoA-I (0.1-10mg), incubate at 37°C for 36 hours, Store at 4°C.
[0043] (2) Observation of recombinant lipoprotein and Aβ by transmission electron microscope 1-40 oligomer binding site
[0044] 20μg / ml recombinant lipoprotein and 200nM Aβ 1-40 The oligomers were incubated in a 37°C incubator for 24 hours, centrifuged and concentrated to 800 μg / ml recombinant lipoprotein, and at the same time, the recombinant lipoprotein and Aβ 1-40 Oligomers were used as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com