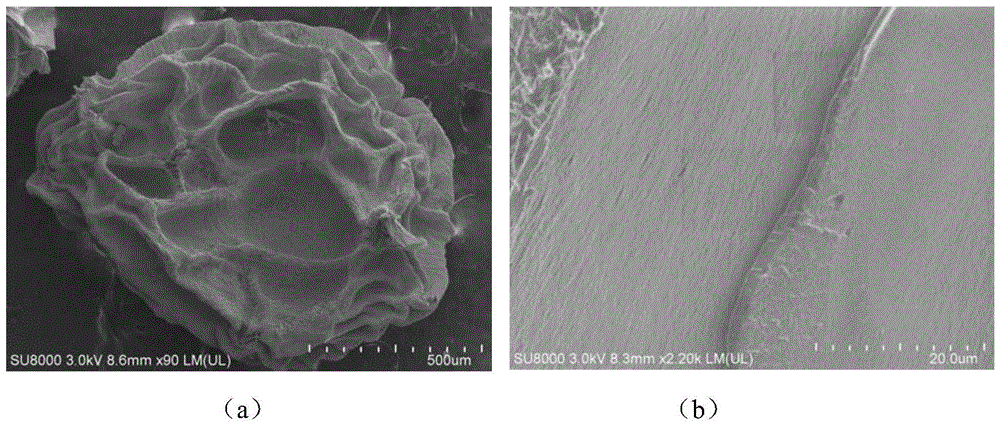
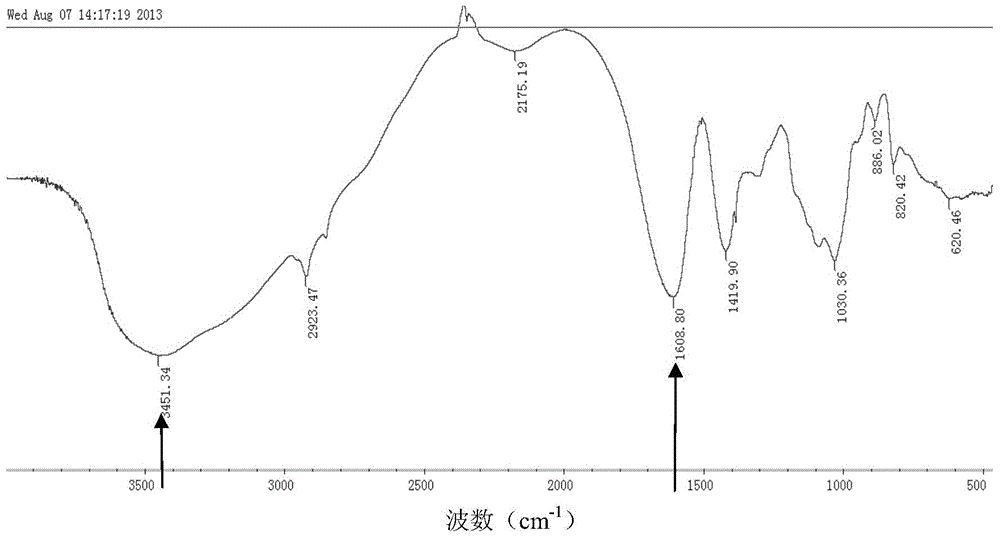
A kind of multi-layer slow-release microsphere preparation loaded with VEGF and vancomycin, preparation method and application
A sustained-release microsphere preparation, vancomycin technology, applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the problem of reducing the therapeutic effect of drugs, increasing the complexity of application, Problems such as low drug loading and encapsulation efficiency can achieve the effect of maintaining drug concentration, meeting the needs of double repair, and avoiding excessive side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Take 20mL of deionized aqueous solution of sodium alginate (SA) with a mass volume ratio of 4.0g: 100mL, stir and mix with 0.4mL sterile aqueous solution containing 0.8ug VEGF, and then add 5.0g: 100mL dropwise 30mL CaCl 2 In the solution, stir at a constant speed of 200r / min for 30min to prepare calcium alginate core microspheres;
[0033] (2) The core sphere that step (1) obtains is added to the mass volume ratio that is dissolved with 100mg vancomycin being stirred and is 3.0g:100mL, 30mL chitosan (CS) dilute acetic acid aqueous solution (the volume of dilute acetic acid aqueous solution Fraction is 1%), stirring speed is 500r / min, and stirring time is 10min, makes calcium alginate-chitosan composite drug-loaded monolayer core microsphere;
[0034] (3) Add the microspheres prepared in step (2) to the sodium alginate solution containing 0ug VEGF mass volume ratio of 1.0g: 100mL, 30mL, 500r / min, and stir for 10min to obtain calcium alginate- Chitosan-sodium algin...
Embodiment 2
[0044] (1) Take 20mL of sodium alginate (SA) solution with a mass volume ratio of 1.0g: 100mL, stir and mix with 0.4mL sterile aqueous solution dissolved in 0.8ug VEGF, and then drop in a mass volume ratio of 15.0g: 100mL, 30mL of CaCl 2 In the solution, stir at a constant speed of 200r / min for 30min to prepare calcium alginate core microspheres;
[0045] (2) The core sphere obtained in step (1) is added to the mass volume ratio of 2.0g:100mL, 30mL of chitosan (CS) dilute acetic acid aqueous solution that is dissolved with 100mg vancomycin being stirred, and the stirring speed is 500r / min, the stirring time is 10min, and the calcium alginate-chitosan composite drug-loaded single-layer core microspheres are prepared;
[0046] (3) Add the microspheres prepared in step (2) to the sodium alginate solution containing 0ug VEGF mass volume ratio of 1.0g: 100mL, 30mL, 500r / min, and stir for 10min to obtain calcium alginate- Chitosan-sodium alginate two-layer microspheres;
[0047]...
Embodiment 3
[0052] (1) Take 20mL of sodium alginate (SA) solution with a mass volume ratio of 4.0g: 100mL, stir and mix with 0.4mL sterile aqueous solution dissolved in 0.8ug VEGF, and then drop in a mass volume ratio of 15.0g: 100mL, 30mL of CaCl 2 In the solution, stir at a constant speed of 200r / min for 30min to prepare calcium alginate core microspheres;
[0053] (2) The core sphere obtained in step (1) is added to the mass volume ratio of 1.0g:100mL, 30mL chitosan (CS) dilute acetic acid aqueous solution that is dissolved with 100mg vancomycin being stirred, and the stirring speed is 500r / min, the stirring time is 10min, and the calcium alginate-chitosan composite drug-loaded single-layer core microspheres are prepared;
[0054] (3) Add the microspheres prepared in step (2) to the sodium alginate solution containing 0ug VEGF with a mass volume ratio of 1.0g: 100mL and 30mL, and stir at a speed of 500r / min for 10min to obtain seaweed Calcium acid-chitosan-sodium alginate two-layer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com