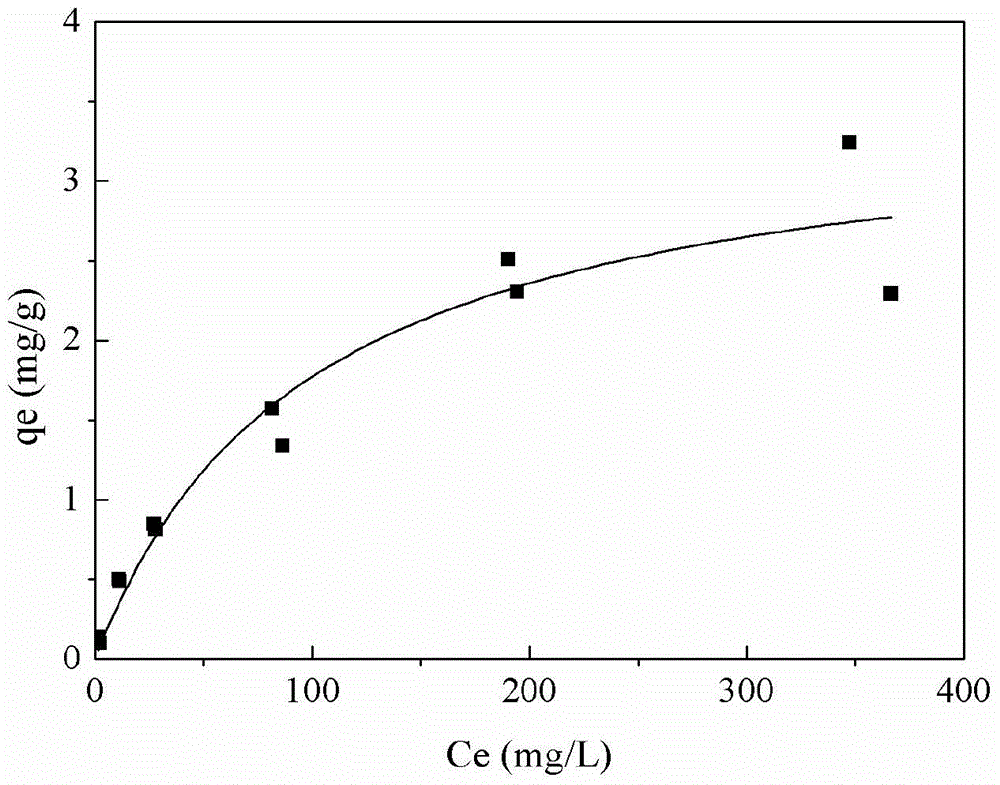
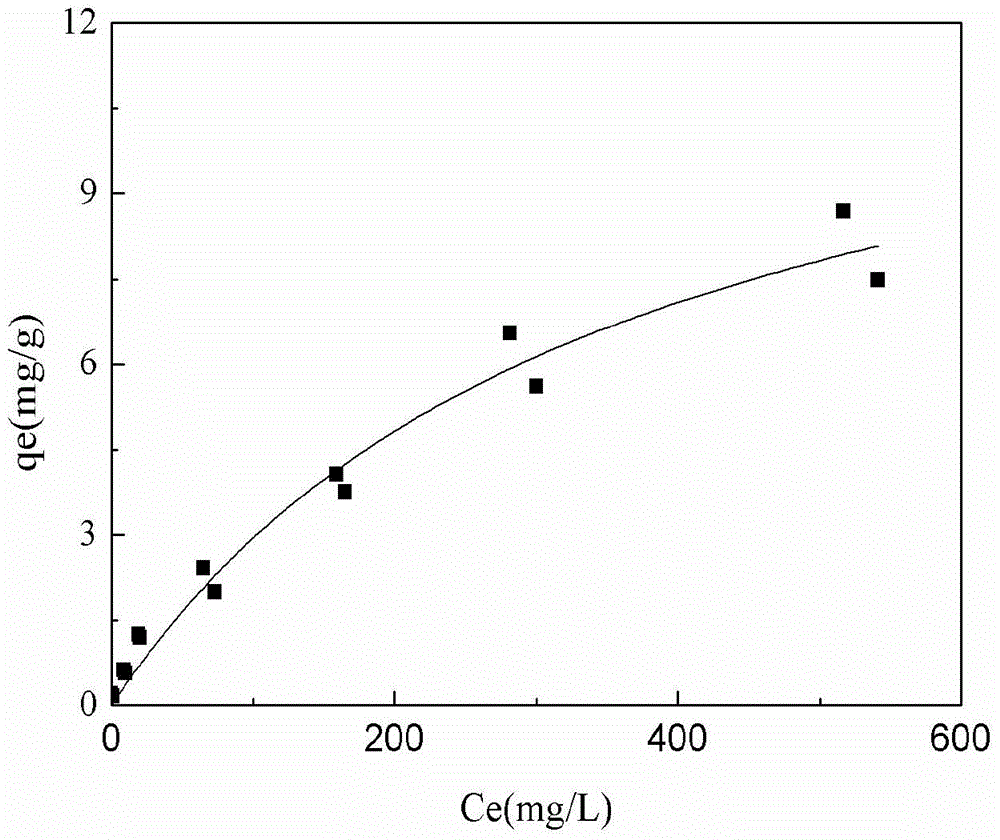
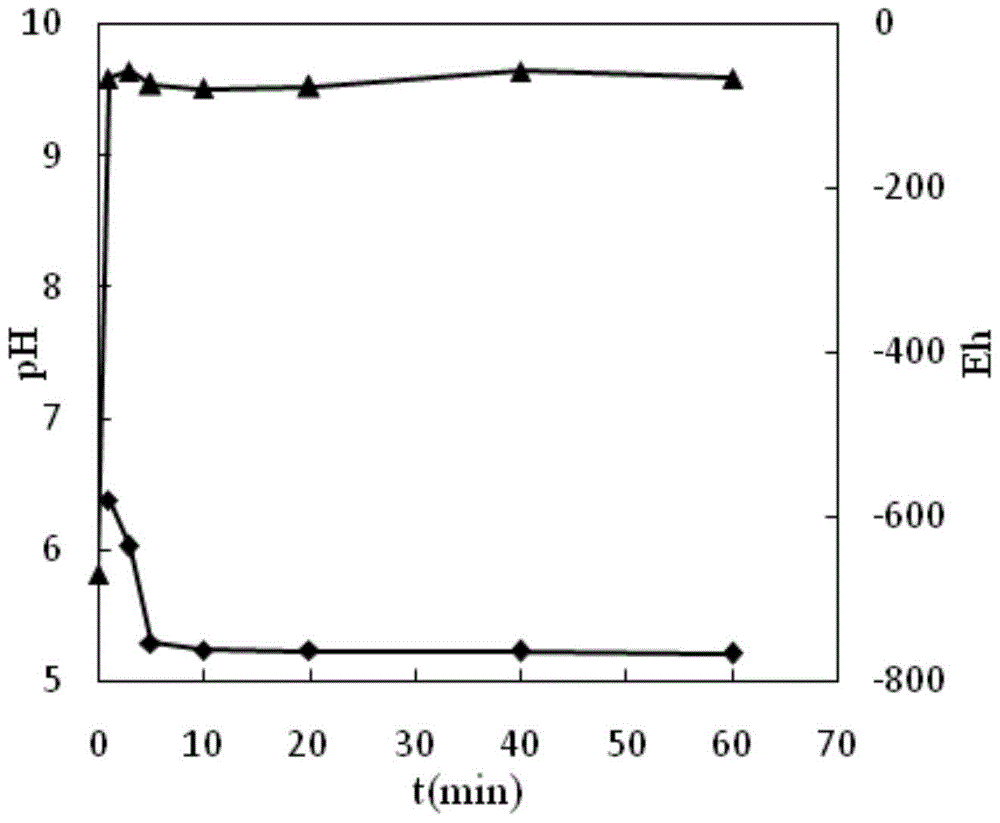
A zeolite-loaded nano-iron material for removing heavy metals in water and its preparation method
A nano-iron and heavy metal technology, applied in the field of environmental engineering and environmental materials, can solve the problems of reduced pollutant removal efficiency, affected reaction activity, difficult PRB reaction medium, etc., to improve reduction potential and water pH value, strong adsorption capacity and The effect of improving reducing power, removal rate and reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]Example 1: The zeolite-loaded nano-iron material of the present invention is formed after nano-iron is loaded on the pores and surfaces of natural zeolite particles collected from Baojiatun, Faku County, Shenyang, with a ratio of parts by weight of 100:1 to 100:1.3. Include the following steps:
[0032] a. Soak the 80-100 mesh above-mentioned natural zeolite in 1mol / L HCl solution for 24 hours, then rinse with deionized water repeatedly for 3-5 times, until the pH value of the aqueous solution reaches 6.0-7.0, then remove the aqueous solution, 70-100℃ Ready to use after drying;
[0033] b. Soak the dried zeolite in 5mmol / L FeCl 3 In solution, zeolite and FeCl 3 The mass-to-volume ratio of the solution is 1:40, shake and react at 25°C and 170rpm / min for 22h, then remove the reaction solution, and then repeatedly wash the zeolite with deionized water for 3 to 5 times, then separate and remove the aqueous solution, and the obtained saturated adsorbed Fe 3+ The zeolite is...
Embodiment 2
[0037] a. Soak 80-100 mesh natural zeolite in 3mol / L HCl solution for 24 hours, then rinse with deionized water repeatedly for 3-5 times, until the pH value of the aqueous solution reaches 6.0-7.0, then remove the aqueous solution, and heat it at 70-100°C Ready to use after drying;
[0038] b. Soak the dried zeolite in 10mmol / L FeCl 3 In solution, zeolite and FeCl 3 The mass-volume ratio of the solution is 1:60, shake and react at 25°C and 170rpm / min for 24h, then remove the reaction solution, and then repeatedly wash the zeolite with deionized water for 3 to 5 times, then separate and remove the aqueous solution, and the obtained saturated adsorbed Fe 3+ The zeolite is transferred to the beaker;
[0039] c. Prepare 1.5mol / L NaBH with 0.1% NaOH solution 4 The solution is ready for use;
[0040] d. will be equipped with the above-mentioned saturated adsorption Fe 3+ The beaker of the zeolite is placed on the shaker, and the prepared NaBH 4 After the solution is added to t...
Embodiment 3
[0042] a. Soak 80-100 mesh natural zeolite in 1mol / L HCl solution for 24 hours, then rinse with deionized water repeatedly for 3-5 times until the pH value of the aqueous solution reaches 6.0-7.0, then remove the aqueous solution, and set the temperature at 70-100°C Ready to use after drying;
[0043] b. Soak the dried zeolite in 10mmol / L FeCl 3 In solution, zeolite and FeCl 3 The mass-to-volume ratio of the solution is 1:100, shake and react at 25°C and 170rpm / min for 26h, then remove the reaction solution, and then repeatedly wash the zeolite with deionized water for 3 to 5 times, then separate and remove the aqueous solution, and the obtained saturated adsorbed Fe 3+ The zeolite is transferred to the beaker;
[0044] c. Prepare 1mol / L NaBH with 0.2% NaOH solution 4 The solution is ready for use;
[0045] d. will be equipped with the above-mentioned saturated adsorption Fe 3+ The beaker of the zeolite is placed on the shaker, and the prepared NaBH 4 After the solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com