Fluorescent silver nucleinate as well as preparation method and application thereof
A fluorescent nucleic acid silver, molecular nucleic acid technology, used in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve iron poisoning, heart and liver diseases, diabetes, difficult to accurately measure metal ions, and cannot be well differentiated Problems such as divalent iron ions and trivalent iron ions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 (best synthesis condition):
[0019] DNA and AgNO 3 The solution was mixed in sodium citrate buffer (pH 5, 10 mM), DNA and Ag +The concentrations were 6.4 and 200 μM, respectively. Transfer the mixed solution to a water bath for heating. The temperature rises to the Tm value of DNA (75°C) and stabilizes for three minutes, then slowly cools down to room temperature for 3.5 hours. 4 - :Ag + =2:1 add NaBH 4 (10 mM) solution, mix well and store in the dark at room temperature for 10 minutes, then transfer to 4 ℃ and store in the dark for 36 hours, the final volume of the reaction solution is 1 mL; prepare 2 μM, 1 μM, 0.5 μM DNA-AgNCs aqueous solution, Excited with 600 nm red light, the measured fluorescence intensity at 660 nm was 135, 72, and 40, respectively.
Embodiment 2
[0021] DNA and AgNO 3 The solution was mixed in sodium citrate buffer (pH 5, 10 mM), DNA and Ag + The concentrations were 6.4 and 100 μM respectively, and the mixed solution was transferred to a water bath for heating. After raising the temperature (70 ℃), it was stable for three minutes, and then slowly cooled to room temperature. The cooling process took 6 hours; 4 - :Ag + =1.5:1 add NaBH 4 (10 mM) solution, mix well and store in the dark at room temperature for 10 minutes, then transfer to 4 ℃ and store in the dark for 30 hours, the final volume of the reaction solution is 1 ml; prepare 2 μM, 1 μM, 0.5 μM DNA-AgNCs aqueous solution, and use Excited with 600 nm red light, the measured fluorescence intensity at 660 nm was 70, 34, and 15, respectively.
Embodiment 3
[0023] DNA and AgNO 3 The solution was mixed in sodium citrate buffer (pH 5, 10 mM), DNA and Ag + The concentrations were 6.4 and 200 μM respectively, and the mixed solution was transferred to a water bath for heating. After the temperature rose to 80 °C, it was stable for three minutes, and then slowly cooled to room temperature. The cooling process lasted for 4 hours; 4 - :Ag + =1:1 add NaBH 4 (10 mM) solution, mix well and store in the dark at room temperature for 10 minutes, then transfer to 4 ℃ and store in the dark for 24 hours, the final volume of the reaction solution is 1 ml; prepare 2 μM, 1 μM, 0.5 μM DNA-AgNCs aqueous solution, use Excited by 600 nm red light, the measured fluorescence intensity at 660 nm was 80, 35, 18, respectively.
[0024] The compound that obtains with embodiment 1 is test compound
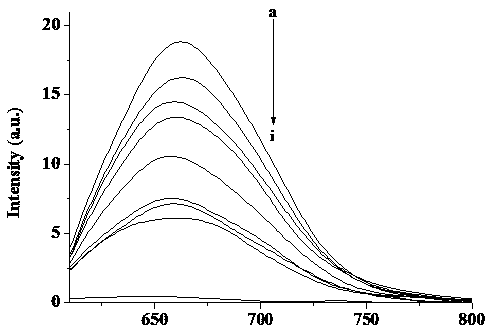
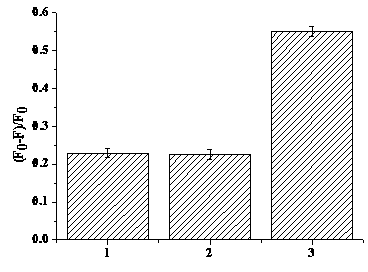
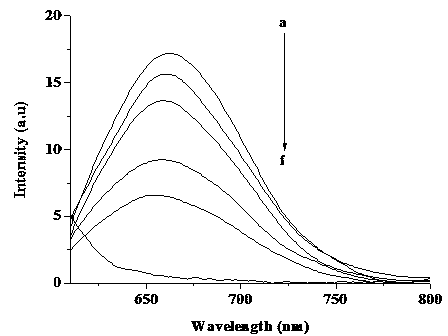
[0025] Select a concentration of 0.5 μM DNA-AgNCs (λex=600 nm, λem=660 nm) aqueous solution, and use Fe(NO 3 ) 3 Titrate with Fe(NO 3 ) 3 As the concent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com