Method for preparing TiO2 nanocrystal particles
A technology of nanocrystalline particles and nanotubes, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc. Monodispersity, high photocatalytic activity, high crystallinity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
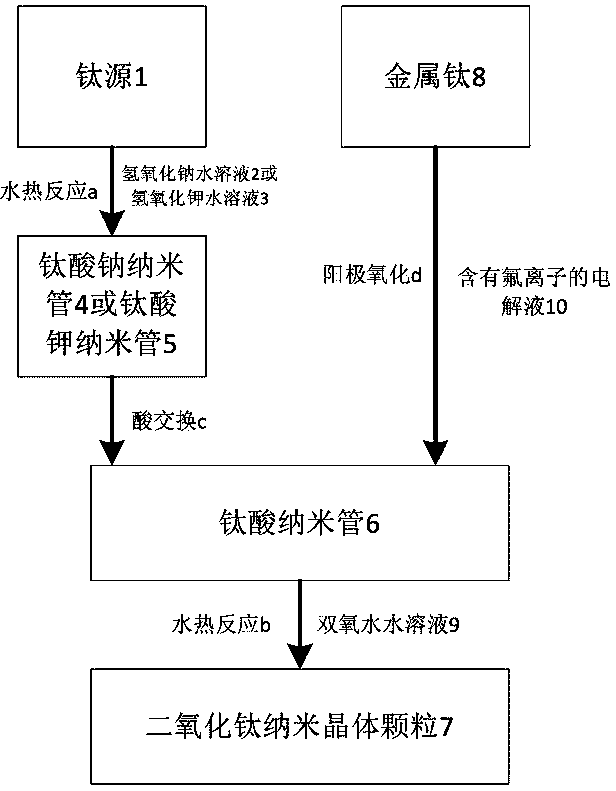
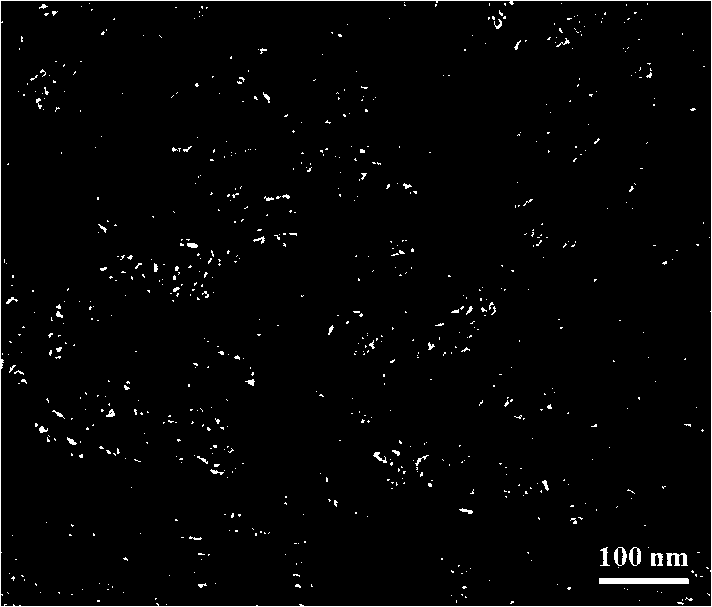
[0055] Take 2 grams of titanium oxide powder and disperse it in 80 milliliters of sodium hydroxide solution with a concentration of 10 moles per liter, then transfer the solution to a hydrothermal kettle and keep the temperature at 120 degrees Celsius for 24 hours to obtain sodium titanate nanotubes. When the temperature of the reaction kettle was lowered to room temperature, the hydrothermal kettle was taken out, the supernatant liquid was poured off, and the white sodium titanate nanotube solid at the bottom was transferred to a beaker, ultrasonically dispersed, washed 5 times with 200 ml of deionized water, and centrifuged. The washed sodium titanate nanotubes were obtained. Adding 500 milliliters of nitric acid solution with a concentration of 0.01 mole per liter into the washed sodium titanate nanotubes, ultrasonication and standing for 6 hours, so that the sodium ions in the sodium titanate nanotubes are completely replaced by hydrogen ions in the nitric acid solution, O...
Embodiment 2
[0057] Take 1 gram of titanium oxide powder and disperse it in 80 milliliters of sodium hydroxide solution with a concentration of 12 moles per liter, then transfer the solution to a hydrothermal kettle and keep the temperature at 100 degrees Celsius for 24 hours to obtain sodium titanate nanotubes. When the temperature of the reaction kettle was lowered to room temperature, the hydrothermal kettle was taken out, the supernatant liquid was poured off, and the white sodium titanate nanotube solid at the bottom was transferred to a beaker, ultrasonically dispersed, washed 5 times with 200 ml of deionized water, and centrifuged. The washed sodium titanate nanotubes were obtained. Adding 200 milliliters of nitric acid solution with a concentration of 0.1 mole per liter into the washed sodium titanate nanotubes, ultrasonication and standing for 6 hours, so that the sodium ions in the sodium titanate nanotubes are completely replaced by hydrogen ions in the nitric acid solution, Obt...
Embodiment 3
[0059] Take 5 grams of titanium sulfate and disperse it in 80 milliliters of sodium hydroxide solution with a concentration of 10 moles per liter, then transfer the solution to a hydrothermal kettle and keep the temperature at 120 degrees Celsius for 24 hours to obtain sodium titanate nanotubes. When the temperature of the reaction kettle was lowered to room temperature, the hydrothermal kettle was taken out, the supernatant liquid was poured off, and the white sodium titanate nanotube solid at the bottom was transferred to a beaker, ultrasonically dispersed, washed 5 times with 200 ml of deionized water, and centrifuged. The washed sodium titanate nanotubes were obtained. Adding 500 milliliters of nitric acid solution with a concentration of 0.01 mole per liter into the washed sodium titanate nanotubes, ultrasonication and standing for 6 hours, so that the sodium ions in the sodium titanate nanotubes are completely replaced by hydrogen ions in the nitric acid solution, Obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com