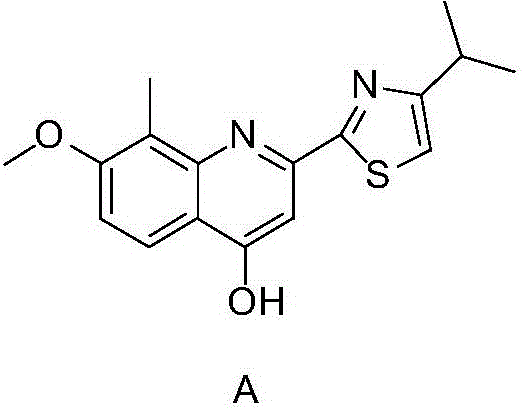
A kind of preparation method of tmc-435 important intermediate
A technology of TMC-435 and intermediates, which is applied in the field of preparation of important intermediates of TMC-435, and can solve problems such as high price, harsh reaction conditions, and complicated post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1: The preparation method of the important intermediate of the anti-hepatitis C drug TMC-435 adopts the following specific process steps.
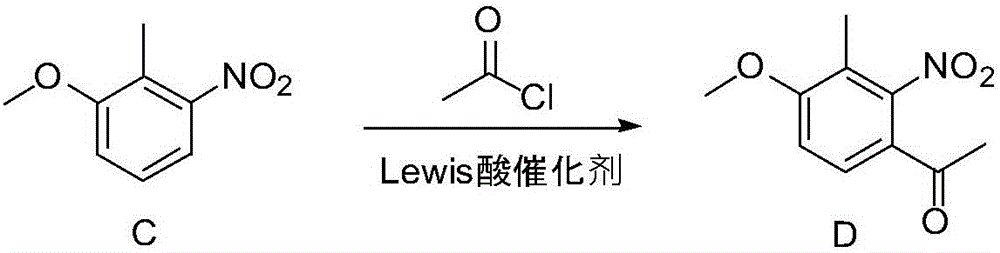
[0090] (1) Synthesis of Compound C:
[0091]
[0092] Prepare 10wt% sodium hydroxide solution (sodium hydroxide 32.70g, 0.0817mol) and join in the reaction flask, add compound B (5.00g, 0.0327mol) and tetrabutylammonium bromide (0.53g, 0.0016mol) successively, After stirring for 0.5 h, dimethyl sulfate (10.30 g, 0.0817 mol) was slowly added dropwise, and after 1.0 h of reaction at room temperature, TLC confirmed that the reaction was complete. Stop the reaction, add sodium hydroxide solution, stir for 0.5 h, filter with suction, wash the filter cake with water (2×50 mL), dry, and decolorize with activated carbon to obtain 5.1 g of yellow solid; yield 93.5%, 1 H-NMR (500MHz, CDCl 3 )δ (ppm): 2.36 (3H, s), 3.89 (3H, s), 7.04 (1H, d), 7.26 (1H, t), 7.39 (1H, d).
[0093] (2) Synthesis of Compound D:
[0094]
[009...
Embodiment 2
[0121] Embodiment 2: The preparation method of the important intermediate of TMC-435 adopts the following specific process steps.
[0122] (1) Synthesis of Compound C:
[0123] Prepare 15% potassium hydroxide solution (36.62g, 0.0981mol) and add it to the reaction flask, add compound B (5.00g, 0.0327mol) and tetrabutylammonium bromide (1.06g, 0.0033mol) in turn, and stir for 0.5h Then, dimethyl sulfate (12.36 g, 0.0981 mol) was slowly added dropwise, the temperature was controlled at about 30° C., and TLC confirmed that the reaction was complete after reacting at room temperature for 1 h. Stop the reaction, add potassium hydroxide solution, stir for 0.5 h, filter with suction, wash the filter cake with water (2×50 mL), dry, and decolorize with activated carbon to obtain 4.97 g of yellow solid; yield 91.0%.
[0124] (2) Synthesis of Compound D:
[0125] Add 80 mL of anhydrous dichloromethane into the reaction flask, add compound C (10.00 g, 0.0600 mol) under stirring, cool do...
Embodiment 3
[0142] Embodiment 3: The preparation method of the important intermediate of TMC-435 adopts the following specific process steps.
[0143] (1) Synthesis of compound C:
[0144] Prepare 30% sodium carbonate solution (46.22g, 0.1308mol) and add it to the reaction flask, then add compound B (5.00g, 0.0327mol) and tetrabutylammonium bromide (3.16g, 0.0098mol) successively, stir slowly after 0.5h Dimethyl sulfate (16.48 g, 0.1308 mol) was added dropwise, and after 2 hours of reaction at room temperature, TLC confirmed that the reaction was complete. Stop the reaction, add sodium carbonate solution, stir for 0.5 h, filter with suction, wash the filter cake with water (2×50 mL), dry, and decolorize with activated carbon to obtain 4.97 g of yellow solid; yield 91.0%.
[0145] (2) Synthesis of compound D:
[0146] Add 80 mL of anhydrous nitromethane into the reaction flask, add compound C (10.00 g, 0.0600 mol) while stirring, cool down to 0 °C with stirring in an ice bath, and add an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com