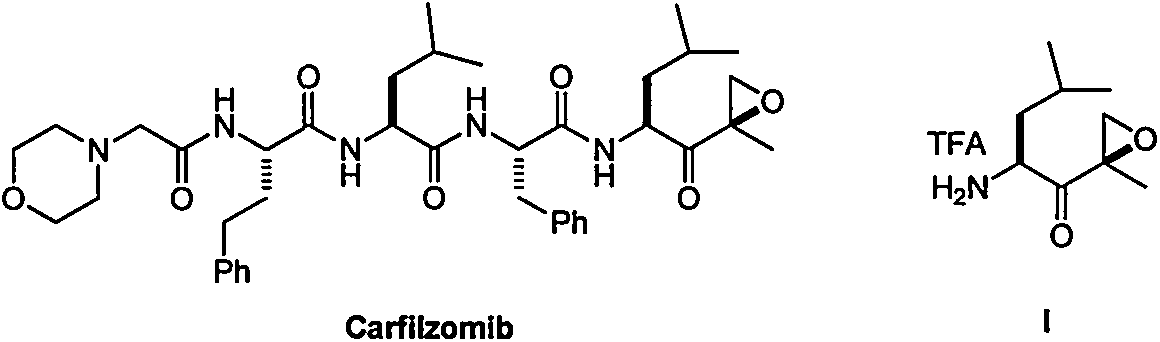
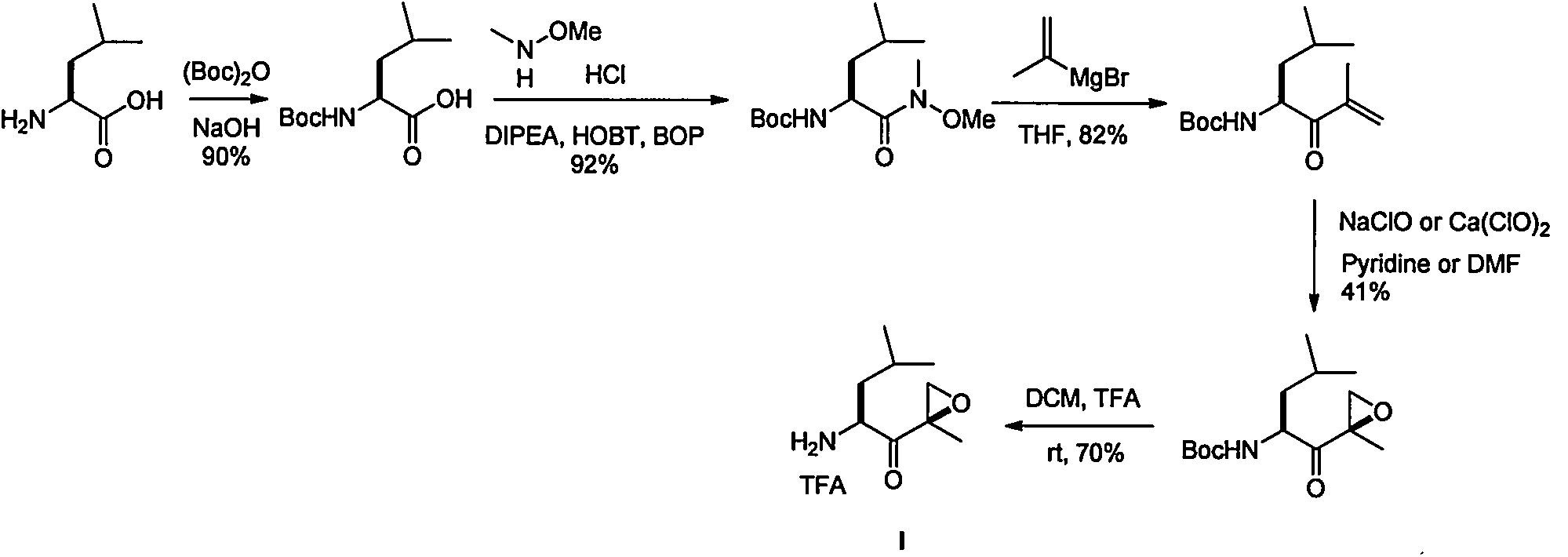
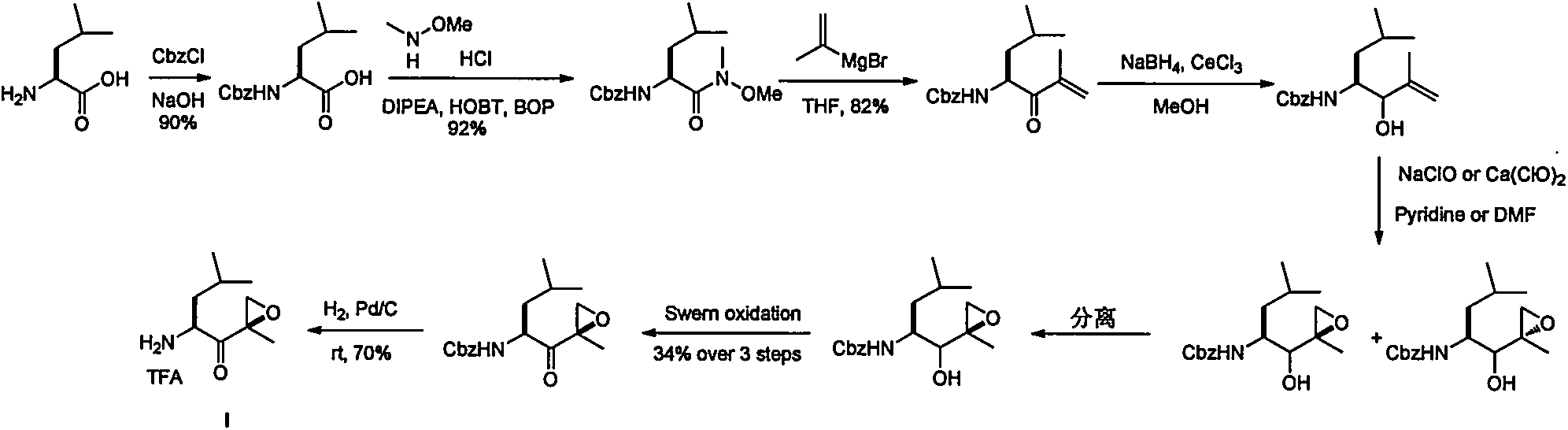
Preparation method of intermediate compounds of carfilzomib and intermediate compounds
A technology for compounds and intermediates, applied in the field of compound preparation, can solve problems such as difficulties in industrialization, and achieve the effects of excellent yield, reduced cost, and improved stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Preparation of N, N-di-tert-butoxycarbonyl-L-leucine
[0054]
[0055] 200g (0.152mol) of L-leucine was dissolved in 500mL of acetonitrile solvent, followed by the addition of 80mL (0.76mol) of diisopropylethylamine, 18.4g (0.152mol) of 4-dimethylaminopyridine and 100g ( 0.456mol) of tert-butoxyformic anhydride, stirred and heated to reflux for 12 hours, TLC detected that the raw material spots disappeared, cooled to room temperature and added water to quench the reaction, added dichloromethane for extraction, the organic phase was washed with saturated brine, anhydrous sulfuric acid After sodium drying, filtration and concentration, 45.2 g of brown solid compound N,N-di-tert-butoxycarbonyl-L-leucine was obtained, with a yield of 90%. The purity is 98.4%.
[0056] 1H NMR (500MHz, DMSO-d6) δppm 12.36[br, 1H,], 3.91-3.87[m, 1H], 1.64-1.61[m, 2H], 1.51-1.47[m, 1H], 1.36[s, 18H] ], 0.87-0.83 [m, 6H].ESI / MS: m / z=332.20(M+H)+.
Embodiment 2
[0057] Example 2: Preparation of (N, N-(di-tert-butoxycarbonyl)-L-leucine-N'-methoxy-N'-formamide
[0058]
[0059] Dissolve N, N-di-tert-butoxycarbonyl-L-leucine (45.2 g, 0.137 mol) in 300 mL of DCM, and add N, O-dimethylhydroxylamine hydrochloride (26.7 g, 0.274 mol), Triethylamine (27.7g, 0.274mol), DMAP (1.7g, 0.014mol), stirred at room temperature for 10 minutes after adding, added EDCl (52.5g, 0.137mmol), stirred at room temperature for 3 hours, and TLC detected that the raw material spots disappeared, and added water The reaction was quenched, extracted with dichloromethane, the organic phase was washed with 1M hydrochloric acid and saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to obtain 47.7g of yellow oily compound (N,N-(di-tert-butoxycarbonyl)-L -Leucine-N'-methoxy-N'-formamide, the yield is 93%. The purity is 96.5%.
[0060] 1H NMR (CDCl3, 500MHz): δppm 4.70 (m, 1H), 3.76, (s, 3H), 3.17, (s, 3H), 1.69, (m, 1H), 1.45-1.37 (m, 2H)...
Embodiment 3
[0061] Example 3: Preparation of (S)-4,4-(di-tert-butoxycarbonylamino)-2,6-dimethyl-1-hepten-3-one
[0062]
[0063] Preparation of 2-propenylmagnesium bromide Grignard reagent: Add magnesium chips (6.7g, 0.279mol) and iodine particles (20mg catalytic amount) to 200mLTHF, heat to reflux, and slowly add 2-bromopropene dropwise after reflux for 1 hour (0.254mol), and then add dropwise about 10mL of iodine, the color disappears, and the reaction is initiated. After the addition of 2-bromopropene is completed, the reaction is refluxed for 2 hours and then cooled to 0-5°C.
[0064] Add (N-(di-tert-butoxycarbonyl)-L-leucine-N′-methoxy-N′-formamide) THF solution (47.7 g in 150 mL THF) dropwise to the prepared Grignard reagent Medium, 0.127mol), after reacting at room temperature for 12 hours, TLC detects that the raw material spots disappear, adding saturated ammonium chloride to quench the reaction, extracting with ethyl acetate, washing the organic phase with saturated brine, dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com