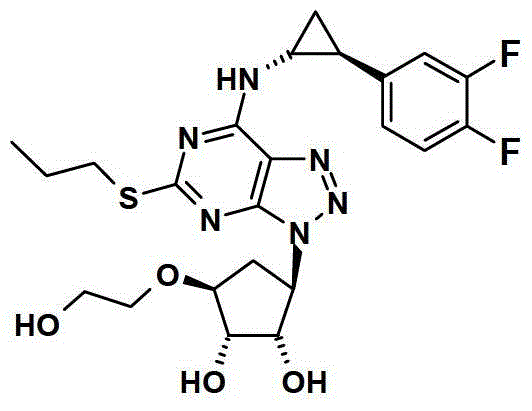
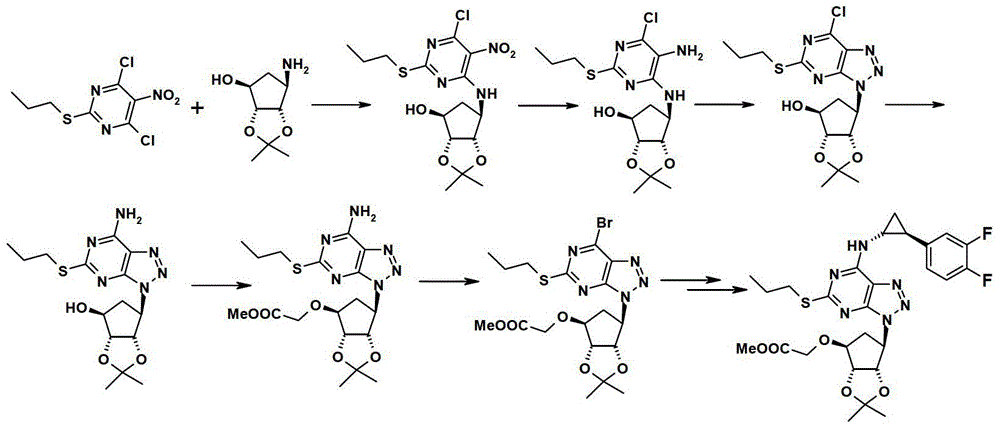
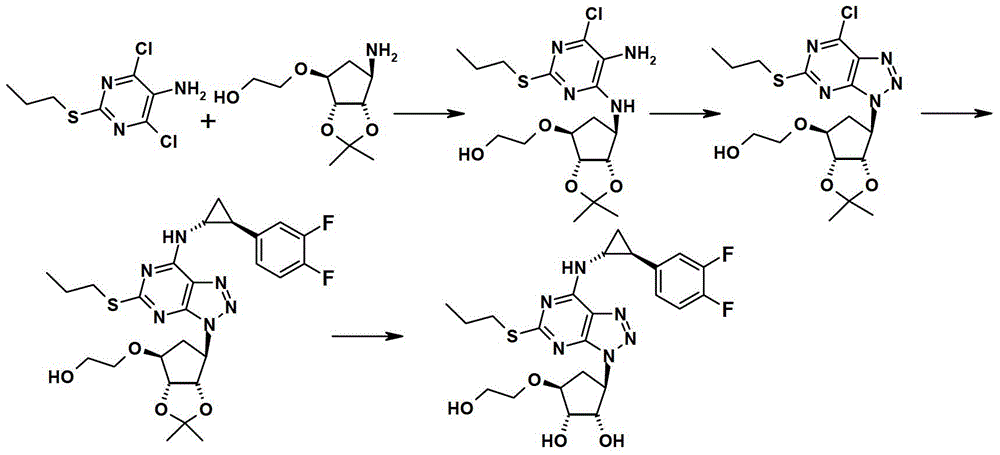
A kind of preparation method of ticagrelor
A technology of ticagrelor and its compound, which is applied in the field of preparation of the small molecule anticoagulant ticagrelor, can solve the problems of being unsuitable for industrial production, and achieve the effects of cost reduction, mild reaction conditions, and shortened reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Add formula II compound (4,6-dichloro-2-(propylthio)pyrimidin-5-amine) (2.68 g, 20 mmol) and dichloromethane (25 ml) into a 100 ml three-necked flask, cool to 0°C, add the compound of formula III ([3αR-(3aα,4α,6α,6aα)]-6-amino-tetrahydro-2,2-dimethyl-4H-cyclopentadiene-1,3-dioxo Heterocyclopent-4-ol) tartrate (3.65 g, 10 mmol), and 10 ml of an aqueous solution of potassium carbonate (4.2 g, 30 mmol) was added dropwise. After the dropwise addition, keep warm at 0°C and stir for 30 minutes, then raise the temperature to room temperature (25°C), and continue stirring for 2 hours. The dichloromethane layer was separated, the water layer was washed with 10 ml of dichloromethane, the dichloromethane layers were combined, dried with anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation, and the residue was separated by column chromatography (eluent: Ethyl acetate: dichloromethane volume ratio = 0-20%: 1 gradient elution), to obtain 1.25 g of pr...
Embodiment 2
[0046] Add compound II (5.4 g, 20 mmol) in a 150 ml three-necked flask, tartrate (7.2 g, 20 mmol) of compound III, dichloromethane (50 ml), cool to -20°C, drop potassium carbonate ( 8.2 g, 30 mmol) in 30 ml of aqueous solution. After the dropwise addition was completed, the temperature was raised to 0° C. and stirred for 2 hours. Separate the dichloromethane layer, dry the dichloromethane layer with anhydrous sodium sulfate, filter, remove the solvent by rotary evaporation, and separate the residue by column chromatography (eluent: ethyl acetate:dichloromethane volume ratio=0- 20%:1 gradient elution), to obtain 2.5 g of product compound IV, based on the amount of compound III, the yield was 28%.
[0047] The product that present embodiment makes confirms to be formula IV compound through detection
Embodiment 3
[0049] Compound II (5.4 g, 20 mmol) and dichloromethane (50 ml) were added to a 150 ml three-necked flask, cooled to 0°C, and 4.1 g of potassium carbonate was added. The tartrate (3.65 g, 10 mmol) of compound III was added to 20 ml of dichloromethane, and 20 ml of an aqueous solution of potassium carbonate (4.1 g, 15 mmol) was added dropwise to prepare the free matter, and the dichloromethane containing the free matter was Layer separation. Slowly add dropwise to the reaction flask of compound II and potassium carbonate, after the dropwise addition is completed, keep warm at 0°C and stir for 2 hours. Separate the dichloromethane layer, dry the dichloromethane layer with anhydrous sodium sulfate, filter, remove the solvent by rotary evaporation, and separate the residue by column chromatography (eluent: ethyl acetate:dichloromethane volume ratio=0- 20%: 1 gradient elution), to obtain 3.5 grams of product compound IV, based on the amount of compound III, the yield was 78%.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com