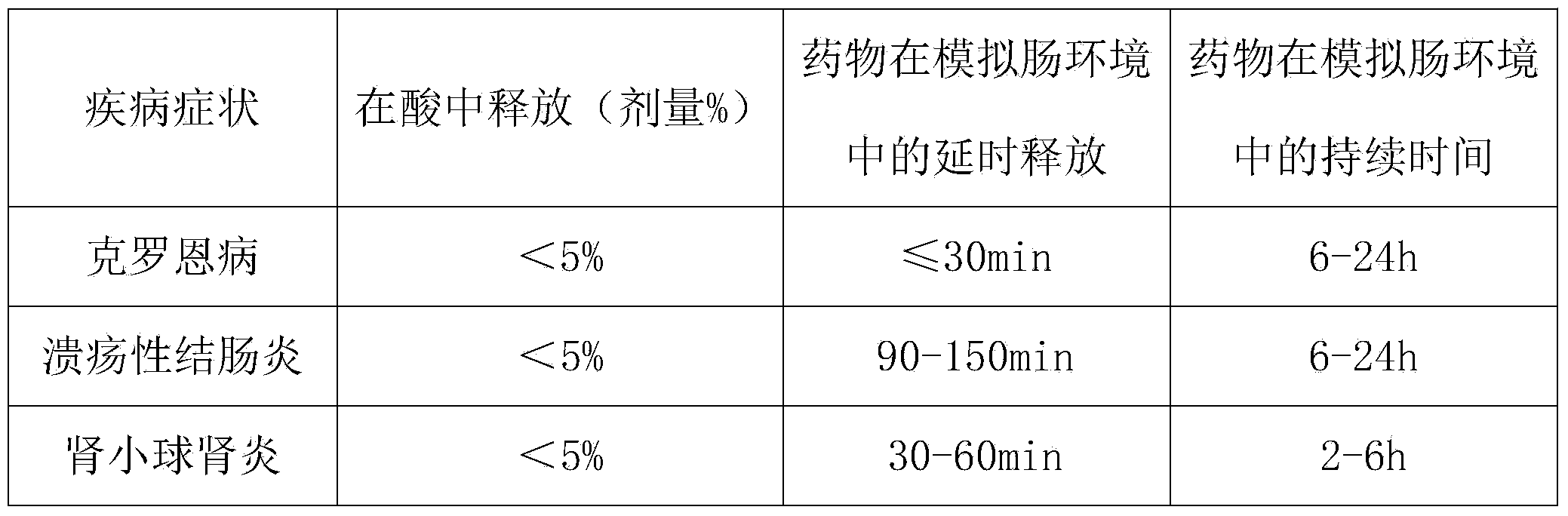
Oral administration composition for delivering salicylic acid medicines to intestinal tract
A composition and drug technology, applied in the directions of drug combination, drug delivery, antipyretic drugs, etc., can solve the problems of affecting the efficacy of drugs, and the efficacy of salicylic acid drugs cannot be sustained for a long time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Preparation of sustained-release sulfasalazine pellets
[0052] (i) Coating solution containing drug
[0053] 242 g of a hydroxypropylmethylcellulose / polyethylene glycol mixture (Colorcon, Dartford, UK) were dissolved in 2850 g of water by vigorous stirring. Then 68 g of micronized sulfasalazine was stirred well in the Opadry solution to form a homogeneous suspension. The suspension was made up with water to a weight of 3160 g.
[0054] (ii) Acidified sealant layer
[0055] 124g of Opadry (as above) and 6g of citric acid (Thornton and Ross) were dissolved in 1450g of water. The solution was made up to 1580 g with water.
[0056] (iii) Application of drug and sealant layers
[0057] The coating chamber of the Aeromatic-Fielder MP-1 fluidized bed coating machine is filled with 4000g of sucrose pellet cores (mesh size of 16-18, Paulaur, USA), wherein the volume of fluidizing air is 80m 3 / h, the inlet temperature is 70° C., the atomization pressure is 29 p...
Embodiment 2
[0061] Example 2: Preparation of Coated Capsules Containing Sulfasalazine Beadlets
[0062] (a) Capsule filling
[0063] The sulfasalazine pellets obtained in Example 1 were filled into No. 0 starch capsules (Capsugel, Greenwood, SC, U.S.): each capsule body was filled with 282 mg pellets, equivalent to 4 mg of sulfasalazine, In a water / isopropanol mixture, seal each capsule body with a cap.
[0065] 47.5 g Eudragit L100 and 15.8 g Eudragit S100 (Degussa, Darmstadt, Germany) were dissolved in a mixture comprising 714 g isopropanol and 24.3 g water. 12.2 g of dibutyl sebacate (plasticizer) and 15.8 g of talc (anti-adherent) were mixed into the coating solution.
[0066] 2400 filled capsules were transferred to the coating pan of a Hi-coater (Vector Corporation, USA) and rotated at 18 rpm. The inlet temperature is set at 40°C and the air flow is 1.16m 3 / min(41ft 3 / min), the capsule was heated for 10 minutes. The coating dispersion was then c...
Embodiment 3
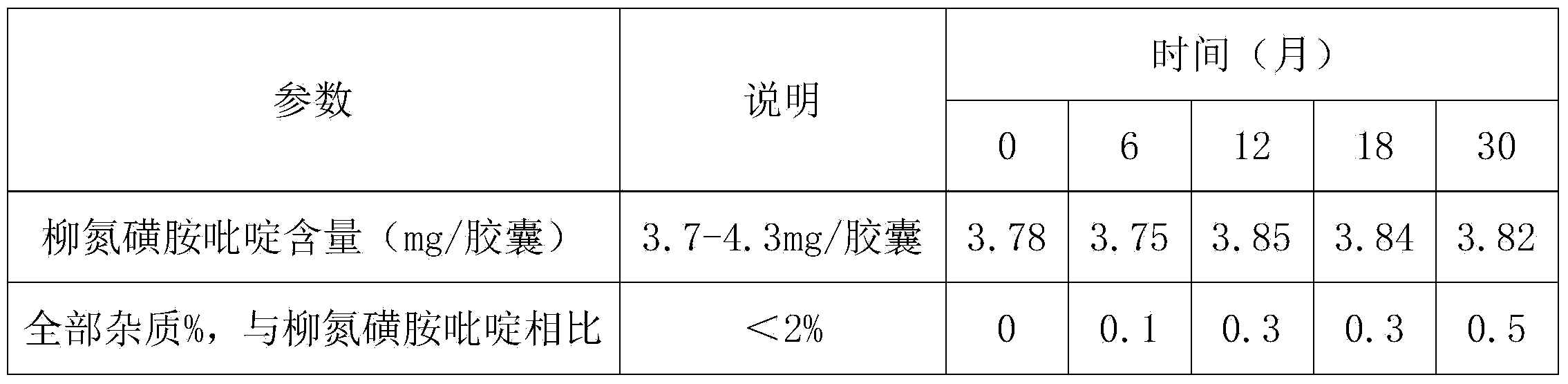
[0069] Example 3: Long-Term Stability of Coated Capsules Containing Sulfasalazine Beadlets
[0070]The table below lists the sulfasalazine and impurity content of capsules prepared in Example 2 and stored for 30 months in HDPE bottles with child-resistant polypropylene caps at 25°C / 60%RH.
[0071]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com