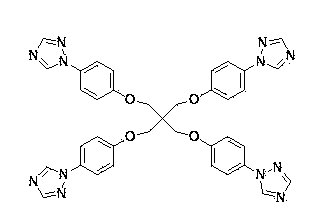
Preparing method and applications of tetra(4-triazolephenyl) pentaerythritol ether
A technology of bromophenylpentaerythritol and pentaerythritol, which is applied in the field of organic synthesis, can solve the problem of unretrieved bibliographical reports and unfounded synthesis methods of tetrakis(4-triazolephenyl)pentaerythritol Tetrakis(4-triazolephenyl)pentaerythritol and other problems, to achieve the effect of simple and easy reaction operation, suitable for large-scale industrial production, and large profit margins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add CuO (0.0398 mg, 0.5 mmol), potassium carbonate (2.0731 g, 15 mmol), triazole (0.345 mg, 5 mmol), p-bromo Phenyl pentaerythritol (0.7 g, 1 mmol), 20 mL DMF. Start stirring at 100 o C, reacted for 24 hours. After the reaction, the reaction liquid was lowered to room temperature, filtered, and 100 mL of water was added to the filtrate, a large amount of precipitate was precipitated, filtered by suction, and the filter cake was collected, with a yield of 78.8%. Anal. Calcd for C 24 h 20 N 10 O: C, 60.73; H, 3.71; N, 25.75. Found: C, 60.83; H, 3.74; N, 25.79.
[0036] Example 2
Embodiment 2
[0038] The molar ratio of p-bromophenyl pentaerythritol: triazole: potassium carbonate: copper oxide is 2:15:30:1
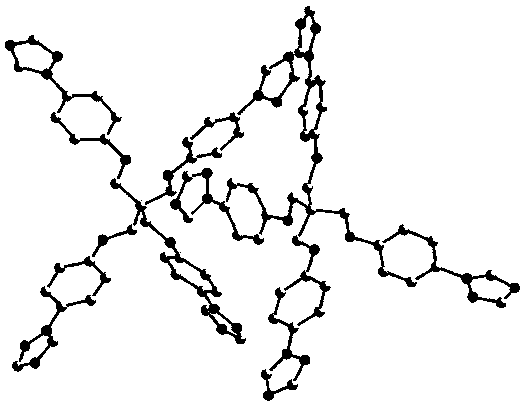
[0039] Add CuO (0.0398 mg, 0.5 mmol), potassium carbonate (2.0731 g, 15 mmol), triazole (0.345 mg, 5 mmol), p-bromo Phenyl pentaerythritol (0.7 g, 1 mmol), 20 mL DMF. Start stirring at 150 oC reacted for 60 hours. After the reaction, the reaction liquid was lowered to room temperature, filtered, and 100 mL of water was added to the filtrate, and a large amount of precipitate was precipitated, filtered with suction, and the filter cake was collected, and recrystallized with water to obtain a single crystal of tetrakis (4-triazolephenyl) pentaerythritol. The rate is 78.8%.
[0040] Anal. Calcd for C 24 h 20 N 10 O: C, 60.73; H, 3.71; N, 25.75. Found: C, 60.83; H, 3.74; N, 25.79.
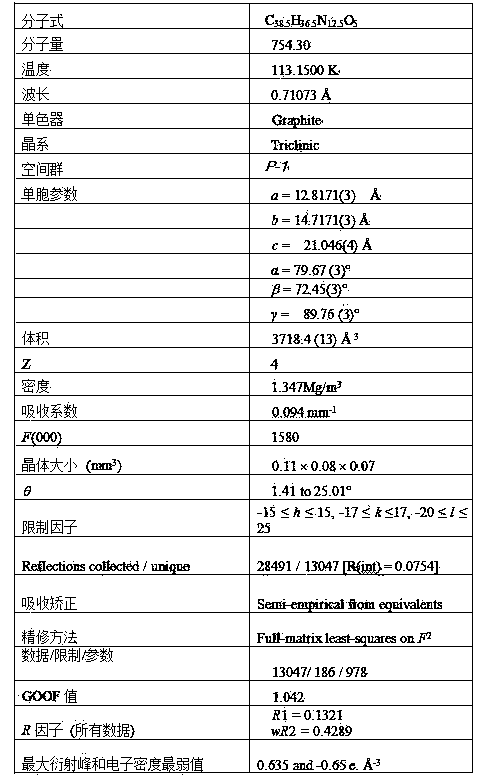
[0041] The crystal structure was determined using an APEX II CCD area detector, using graphite monochromatized Mokα rays (l = 0.71073 ?) as the incident radiation, and w-2q Diffr...
Embodiment 3
[0047] Compound (I), compound (II), potassium carbonate and copper oxide are prepared by reacting under heating conditions; wherein the molar ratio of p-bromophenyl pentaerythritol: triazole: potassium carbonate: copper oxide is 2:15:30 : 1; the reaction temperature is 100° C., and the reaction time is 24 hours; the polar solvent is N,N-dimethylformamide, and the base is sodium hydroxide.
[0048]
[0049]
[0050] Example 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com