Synthesis method of hyperbranched polymers and modification of epoxy curing product by hyperbranched polymers
A hyperbranched polymer and a series of technologies, applied in the field of organic polymers, can solve the problems of single structure and complicated synthesis steps, and achieve the effects of wide source of raw materials, simple synthesis method, and increase of glass transition temperature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
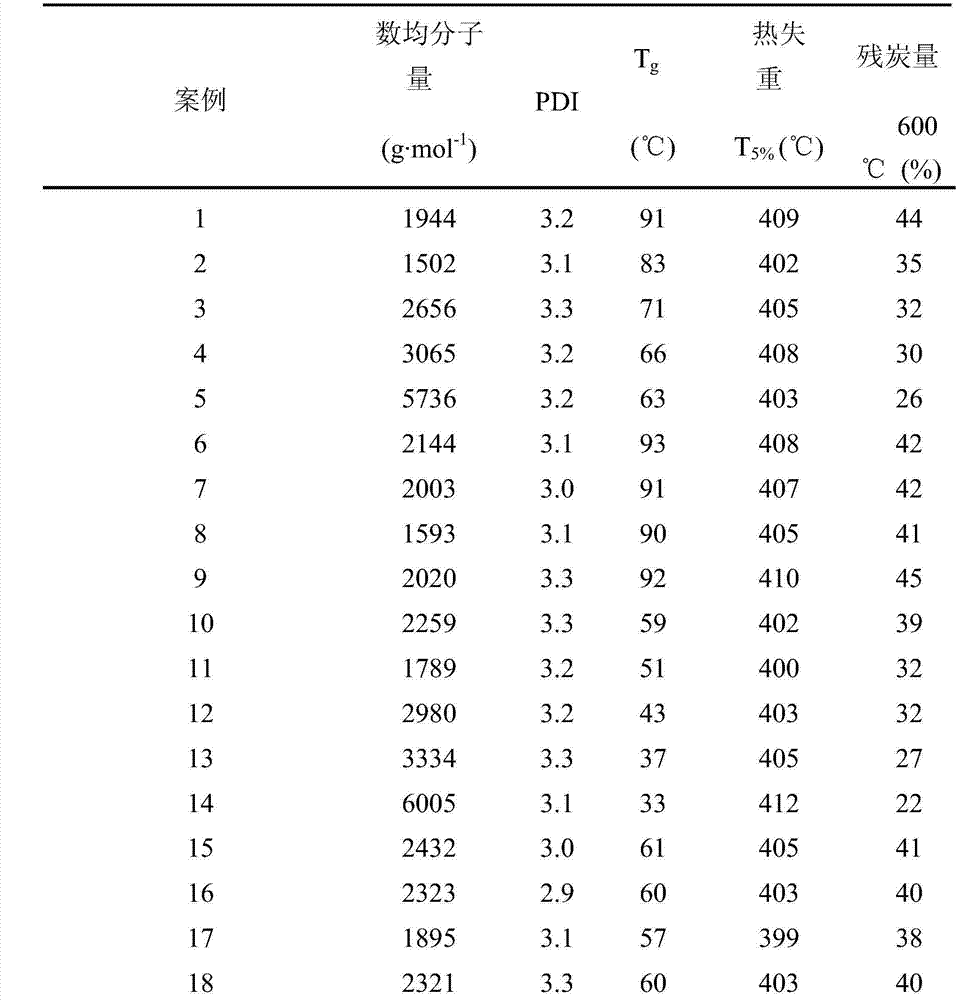
Examples
Embodiment example 1
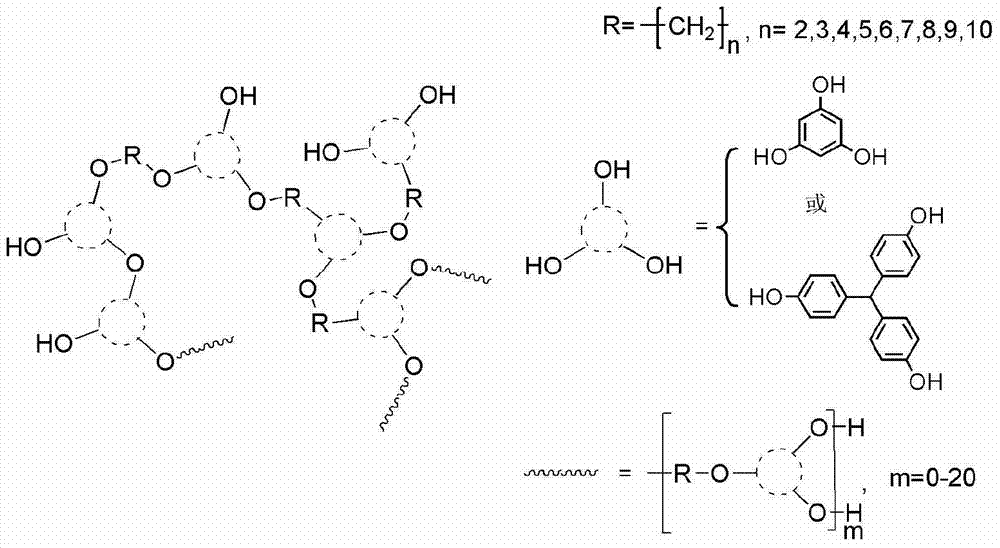
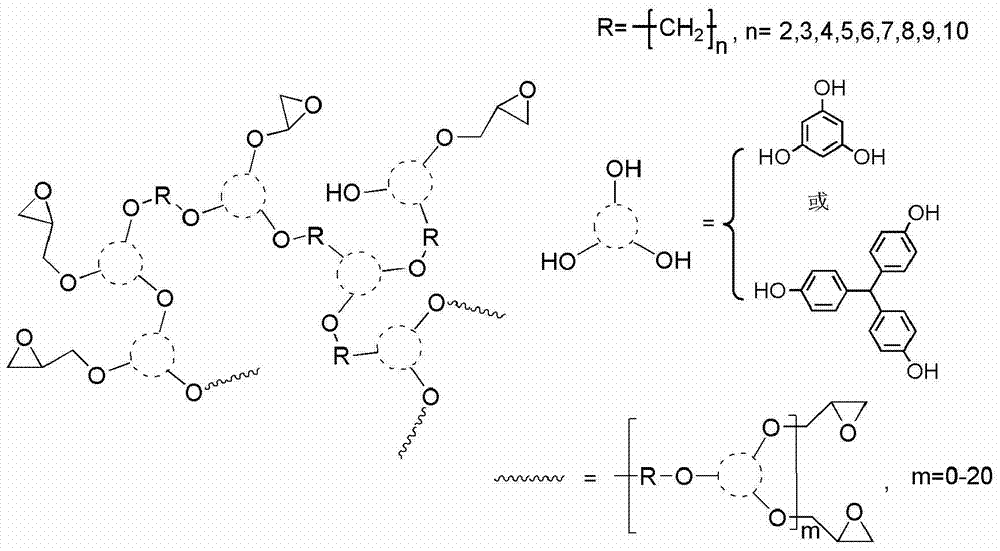
[0033] With 150.0g (0.51mol) of trisphenol methane, 212.8g (1.54mol) of catalyst potassium carbonate, 4.5g of potassium iodide, 83.3g (0.39mol) of 1,4 dibromobutane, simultaneously add in the reactor and use 600mL of N,N dimethylformamide was dissolved; the system was reacted at 80°C for 4 hours; after the reaction, the precipitate was filtered and acidified with hydrochloric acid to obtain the filtrate; the filtrate was precipitated with a mixture of ethanol and water in an equal volume ratio, and dried to obtain 132.6g red solid powder, yield 71%.
Embodiment example 2
[0035] In this embodiment, in the preparation process of the polyether hyperbranched polymer whose end group is a phenolic hydroxyl group, the raw material 150.0g (0.51mol) of trisphenol methane is replaced by 64.3g (0.51mol) of poroglucinol, and other examples 1 is the same.
Embodiment example 3
[0037] In this embodiment, in the preparation process of the polyether hyperbranched polymer whose end group is a phenolic hydroxyl group, the raw material 83.3g (0.39mol) of 1,4-dibromobutane is replaced by 95.1g (0.39mol) of 1,6-dibromohexyl Alkane, others are identical with embodiment case 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com