Methylnaltrexone compound, oral tablet and preparation methods of two
A technology of methylnaltrexone and oral tablets, which is applied in the field of medicine, can solve the problems of low oil-water partition coefficient and low absorption of methylnaltrexone bromide, achieve good oral absorption and improve fat solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] This example is used to illustrate brominated methylnaltrexone phospholipid complex and its preparation method.
[0067] Weigh 100mg of methylnaltrexone bromide, dissolve it in 50ml of methanol at 25°C, add hydrogenated soybean lecithin (HSPC) equivalent to 1 times the molar amount of bromide methylnaltrexone, and disperse evenly. After oscillating at a constant temperature for 5 hours under the same conditions, stop the reaction to obtain a colorless to light yellow clear solution, then evaporate the methanol under reduced pressure at 50°C, dry it in vacuum at 50°C for 48 hours, crush the dried product and sieve it to obtain methyl bromide Naltrexone Phospholipid Complex.
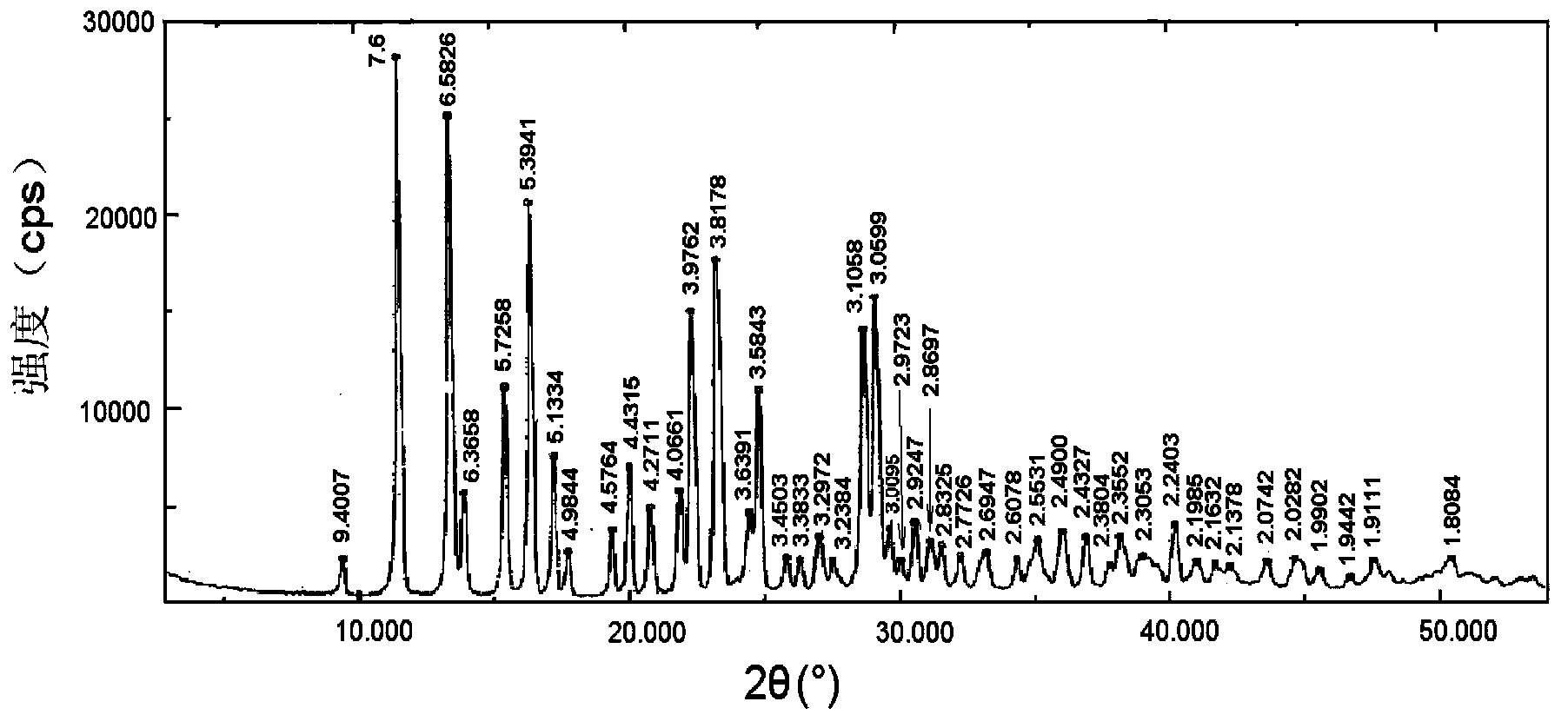
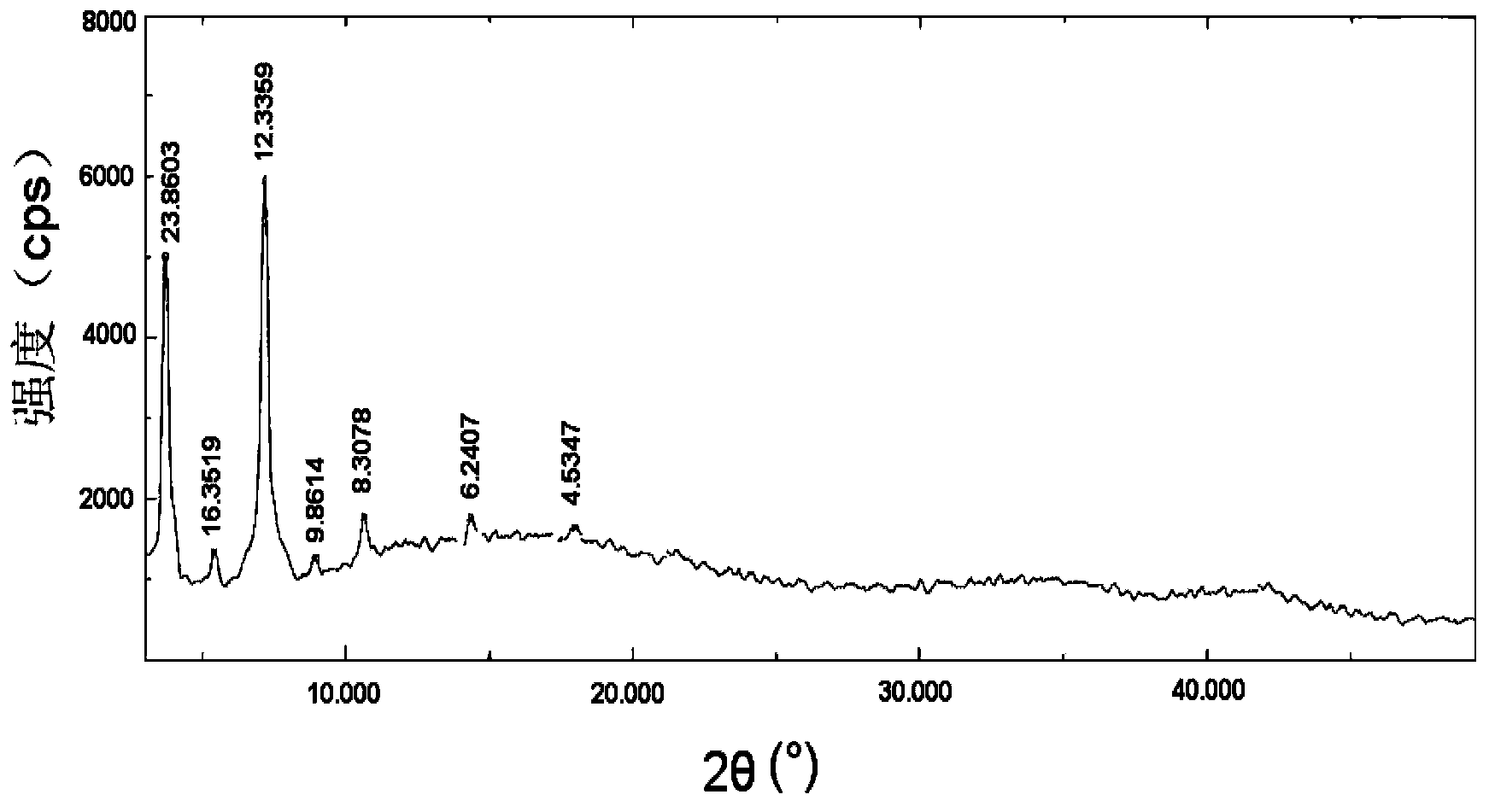
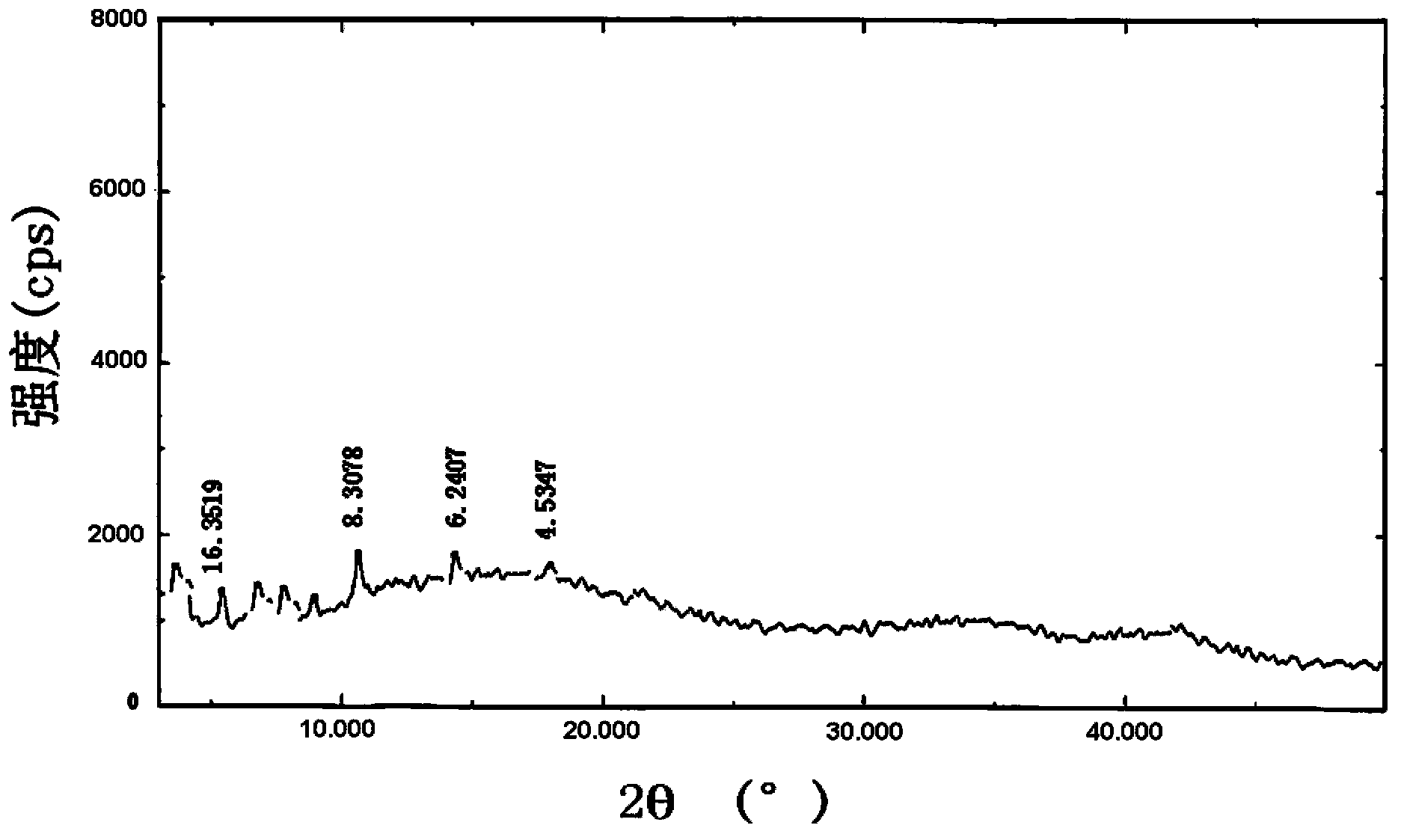
[0068] Take brominated methylnaltrexone, hydrogenated soybean lecithin, brominated methylnaltrexone phospholipid complex, and a physical mixture of brominated methylnaltrexone and hydrogenated soybean lecithin (mixed at a molar ratio of 1:1) Carried out X-ray diffraction analysis, the results are a...
Embodiment 2
[0071] This example is used to illustrate brominated methylnaltrexone phospholipid complex and its preparation method.
[0072] Weigh 50mg of methylnaltrexone bromide, dissolve it in 30ml of ethanol at 30°C, add HSPC equivalent to 2 times the molar amount of bromide methylnaltrexone, disperse evenly, shake at 30°C for 3 hours , to stop the reaction, to obtain a colorless to light yellow clear solution, then evaporate the methanol under reduced pressure at 45°C, dry in vacuum at 50°C for 40h, crush and sieve the dried product to obtain brominated methylnaltrexone phospholipid complex .
Embodiment 3
[0074] This example is used to illustrate brominated methylnaltrexone phospholipid complex and its preparation method.
[0075] Weigh 200mg of methylnaltrexone bromide, dissolve it in 30ml of ethanol at 40°C, add DSPG equivalent to 0.5 times the molar amount of methylnaltrexone bromide, disperse evenly, shake at 40°C for 2 hours , to stop the reaction, to obtain a colorless to pale yellow clear solution, then evaporate methanol under reduced pressure at 55°C, dry in vacuum at 50°C for 36h, crush and sieve the dried product to obtain brominated methylnaltrexone phospholipid complex .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com