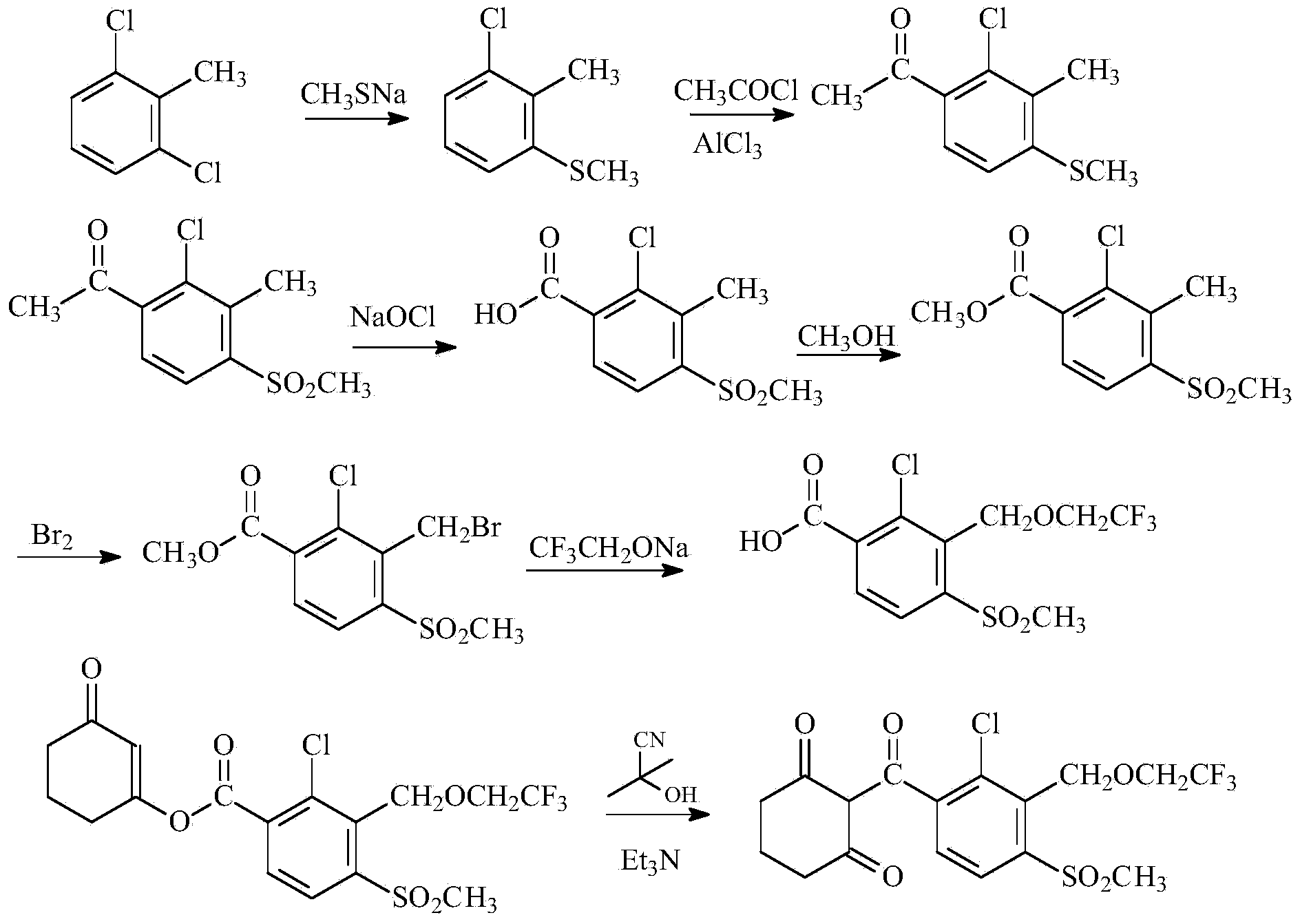
Process for synthesizing triketone herbicide cyclic sulcotrione
A technology of sulfotrione and synthesis process, which is applied in the field of preparation of pesticide technical materials, can solve the problems of high production cost, expensive brominating agent, difficult to handle mercaptan, etc., and achieve reduction of production cost, shortening of reaction steps and time, The effect of production process safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
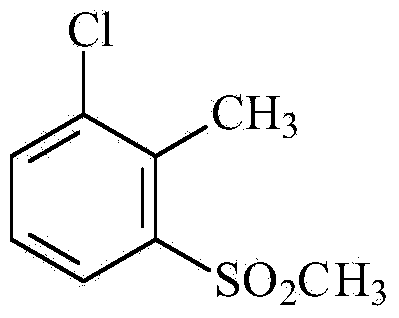
[0033] 1. Synthesis of 2-chloro-6-methylsulfonyltoluene
[0034]
[0035] Add 25.3g (0.2mol) of 2-chlorotoluene, 40.5g (0.3mol) of anhydrous aluminum trichloride and 1,2-dichloroethane into a 500ml four-necked reaction flask equipped with a stirrer, a thermometer and a reflux condenser 150ml of alkane, cooled to 5°C, then added dropwise 25.25g (0.22mol) of methanesulfonyl chloride, the dropping temperature did not exceed 10°C, after the dropwise addition was completed, it was raised to room temperature and continued to react for 10h, followed by LC (liquid chromatography) until the reaction was complete , the reactants were poured into ice water, the organic layer was separated, the aqueous layer was extracted with 1,2-dichloromethane, the organic phases were combined and concentrated to obtain 28.6 g of light yellow solid, with a yield of 84%.
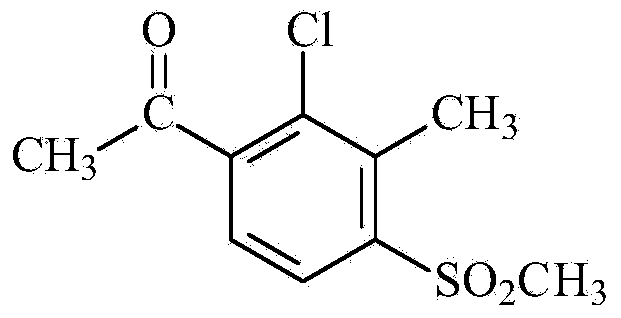
[0036] 2. 2-Chloro-3-acetyl-6-methylsulfonyltoluene
[0037]
[0038] Add 34.0 g (0.2 mol) of 2-chloro-6-methylsulfonyltoluen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com