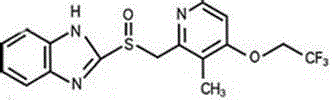
Lansoprazole special superfine powder freeze-dried preparation and preparing method thereof
A technology of lansoprazole and ultra-fine powder, applied in the field of medicine, can solve the problems of low purity, poor stability, easy allergy and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
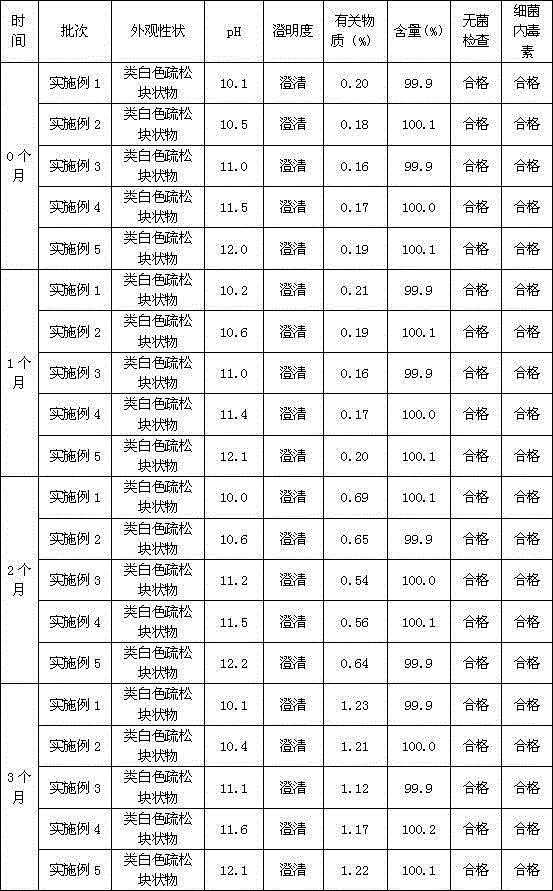
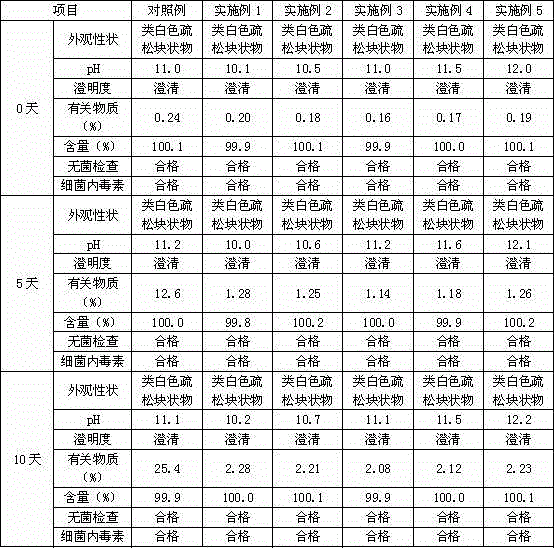
[0027] Mix 300 grams of commercially available lansoprazole crude product (purity 96.5%) and 1500 grams (1900 ml) of absolute ethanol, heat up to 40°C, heat and stir for 40 minutes until the material is completely dissolved, add 15 grams of activated carbon for decolorization for 40 minutes, Remove activated carbon by hot filtration, add 900ml of water for injection dropwise, cool down to 15°C after adding, and keep warm for 3 hours to obtain a white solid precipitate, filter, and rinse the filter cake with 150g (190ml) of absolute ethanol each time, rinse 3 times , to obtain the refined wet product of lansoprazole by suction filtration. Vacuum drying at a temperature of 25° C. for 6 hours gave 271 grams of lansoprazole fine product with a purity of 99.8%.
[0028] The dried lansoprazole refined product is pulverized into superfine powder with two-stage counter-jet jet milling device. The specific process of ultra-fine grinding is as follows: the dry lansoprazole product is p...
Embodiment 2
[0031] Mix 300 grams of commercially available lansoprazole crude product (purity 96.5%) and 1650 grams (2090 ml) of absolute ethanol, heat up to 38 ° C, keep stirring for 30 minutes until the material is completely dissolved, add 22.5 grams of activated carbon and decolorize for 30 minutes, Remove activated carbon by hot filtration, add 1050ml of water for injection dropwise, cool down to 12°C after adding, and keep warm for 3 hours to obtain a white solid precipitate, filter, and rinse the filter cake with 225 grams (285ml) of absolute ethanol each time, rinse twice , to obtain the refined wet product of lansoprazole by suction filtration. Vacuum-dried at 30 DEG C for 5 hours to obtain 270 grams of lansoprazole fine product with a purity of 99.9%.
[0032] The dried lansoprazole refined product is pulverized into superfine powder with two-stage counter-jet jet milling device. The specific process of ultra-fine grinding is as follows: the dry lansoprazole product is passed t...
Embodiment 3
[0035] Mix 300 grams of commercially available lansoprazole crude product (purity 96.5%) and 1800 grams (2280 ml) of absolute ethanol, heat up to 37 ° C, keep stirring for 35 minutes until the material is completely dissolved, add 30 grams of activated carbon and keep it warm for 35 minutes to decolorize. Remove activated carbon by hot filtration, add 1200ml of water for injection dropwise, cool down to 10°C after adding, and keep warm for 2.5 hours to obtain a white solid precipitate, filter, and rinse the filter cake with 300g (380ml) of absolute ethanol each time, rinse twice , to obtain the refined wet product of lansoprazole by suction filtration. Vacuum drying for 4 hours at a temperature of 35°C gave 269 grams of lansoprazole fine product with a purity of 99.9%.
[0036] The dried lansoprazole refined product is pulverized into superfine powder with two-stage counter-jet jet milling device. The specific process of ultra-fine grinding is as follows: the dry lansoprazole...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com